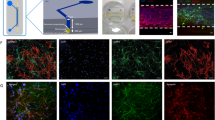
Abstract
Neuroinflammation has either beneficial or detrimental effects, depending on risk factors and neuron–glia interactions in neurological disorders. However, studying neuroinflammation has been challenging due to the complexity of cell–cell interactions and lack of physio-pathologically relevant neuroinflammatory models. Here, we describe our three-dimensional microfluidic multicellular human neural culture model, referred to as a ‘brain-on-a-chip’ (BoC). This elucidates neuron–glia interactions in a controlled manner and recapitulates pathological signatures of the major neurological disorders: dementia, brain tumor and brain edema. This platform includes a chemotaxis module offering a week-long, stable chemo-gradient compared with the few hours in other chemotaxis models. Additionally, compared with conventional brain models cultured with mixed phenotypes of microglia, our BoC can separate the disease-associated microglia out of heterogeneous population and allow selective neuro–glial engagement in three dimensions. This provides benefits of interpreting the neuro–glia interactions while revealing that the prominent activation of innate immune cells is the risk factor leading to synaptic impairment and neuronal loss, validated in our BoC models of disorders. This protocol describes how to fabricate and implement our human BoC, manipulate in real time and perform end-point analyses. It takes 2 d to set up the device and cell preparations, 1–9 weeks to develop brain models under disease conditions and 2–3 d to carry out analyses. This protocol requires at least 1 month training for researchers with basic molecular biology techniques. Taken together, our human BoCs serve as reliable and valuable platforms to investigate pathological mechanisms involving neuroinflammation and to assess therapeutic strategies modulating neuroinflammation in neurological disorders.
Key points
-
This protocol describes the fabrication of a microfluidic device and its implementation in three-dimensional human neural cultures, allowing the study of neuron–glia interactions in neuroinflammation and neurodegeneration.
-
Compared with conventional animal models, this system enables precise reconstitution of key physiological features of neuroinflammation found in patient tissues. In addition, compared with conventional well plates, this model achieves the separation of reactive microglia instead of heterogeneous populations.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Kang, Y. J., Diep, Y. N., Tran, M. & Cho, H. Therapeutic targeting strategies for early- to late-staged Alzheimer’s disease. Int. J. Mol. Sci. 21, 9591 (2020).
Heneka, M. T. et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 14, 388–405 (2015).
Park, J. et al. A 3D human triculture system modeling neurodegeneration and neuroinflammation in Alzheimer’s disease. Nat. Neurosci. 21, 941–951 (2018).
Kinney, J. W. et al. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimers Dement. 4, 575–590 (2018).
Tan, H.-Y., Cho, H. & Lee, L. P. Human mini-brain models. Nat. Biomed. Eng. 5, 11–25 (2021).
Kang, Y. J. & Cho, H. Human brain organoids in Alzheimer’s disease. Organoid 1, e5 (2021).
Keren-Shaul, H. et al. A unique microglia type associated with restricting development of Alzheimer’s disease. Cell 169, 1276–1290.e17 (2017).
McQuade, A. et al. Gene expression and functional deficits underlie TREM2-knockout microglia responses in human models of Alzheimer’s disease. Nat. Comm. 11, 5370 (2020).
Lee, C. Y. D. & Landreth, G. E. The role of microglia in amyloid clearance from the AD brain. J. Neural Transm. 117, 949–960 (2010).
Gui, X. et al. Botulinum toxin type A promotes microglial M2 polarization and suppresses chronic constriction injury-induced neuropathic pain through the P2X7 receptor. Cell Biosci. 10, 45 (2020).
Pitanga, B. P., Nascimento, R. P., Silva, V. D. & Costa, S. L. The role of astrocytes in metabolism and neurotoxicity of the pyrrolizidine alkaloid monocrotaline, the main toxin of Crotalaria retusa. Front. Pharmacol. 3, 144 (2012).
Kang, Y. J., Tan, H.-Y., Lee, C. Y. & Cho, H. An air particulate pollutant induces neuroinflammation and neurodegeneration in human brain models. Adv. Sci. 8, 2101251 (2021).
Waltl, I. & Kalinke, U. Beneficial and detrimental functions of microglia during viral encephalitis. Trends Neurosci. 45, 158–170 (2022).
Chun, H. et al. Severe reactive astrocytes precipitate pathological hallmarks of Alzheimer’s disease via H2O2− production. Nat. Neurosci. 23, 1555–1566 (2020).
Li, Q. & Barres, B. A. Microglia and macrophages in brain homeostasis and disease. Nat. Rev. Immunol. 18, 225–242 (2018).
Volterra, A. & Meldolesi, J. Astrocytes, from brain glue to communication elements: the revolution continues. Nat. Rev. Neurosci. 6, 626–640 (2005).
Masuda, T., Sankowski, R., Staszewski, O. & Prinz, M. Microglia heterogeneity in the single-cell era. Cell Rep. 30, 1271–1281 (2020).
Tan, Y.-L., Yuan, Y. & Tian, L. Microglial regional heterogeneity and its role in the brain. Mol. Psychiatry 25, 351–367 (2020).
Cho, H. et al. Microfluidic chemotaxis platform for differentiating the roles of soluble and bound amyloid-β on microglial accumulation. Sci. Rep. 3, 1823 (2013).
Kim, Y. H. et al. A 3D human neural cell culture system for modeling Alzheimer’s disease. Nat. Protoc. 10, 985–1006 (2015).
Jairaman, A. et al. TREM2 regulates purinergic receptor-mediated calcium signaling and motility in human iPSC-derived microglia. eLife 11, e73021 (2022).
Tran, V. T. A., Kang, Y. J., Kim, H.-K., Kim, H.-R. & Cho, H. Oral pathogenic bacteria-inducing neurodegenerative microgliosis in human neural cell platform. Int. J. Mol. Sci. 22, 6925 (2021).
Sofroniew, M. V. Astrocyte reactivity: subtypes, states, and functions in CNS innate immunity. Trends Immunol. 41, 758–770 (2020).
Linnerbauer, M., Wheeler, M. A. & Quintana, F. J. Astrocyte crosstalk in CNS inflammation. Neuron 108, 608–622 (2020).
Zehntner, S. P. et al. Neutrophils that infiltrate the central nervous system regulate T cell responses. J. Immunol. 174, 5124–5131 (2005).
Sas, A. R. et al. A new neutrophil subset promotes CNS neuron survival and axon regeneration. Nat. Immunol. 21, 1496–1505 (2020).
Spiteri, A. G., Wishart, C. L., Pamphlett, R., Locatelli, G. & King, N. J. C. Microglia and monocytes in inflammatory CNS disease: integrating phenotype and function. Acta Neuropathol. 143, 179–224 (2022).
Jankowsky, J. L. & Zheng, H. Practical considerations for choosing a mouse model of Alzheimer’s disease. Mol. Neurodegener. 12, 89 (2017).
Drummond, E. & Wisniewski, T. Alzheimer’s disease: experimental models and reality. Acta Neuropathol. 133, 155–175 (2017).
Vignal, C. et al. Effects of urban coarse particles inhalation on oxidative and inflammatory parameters in the mouse lung and colon. Part. Fibre Toxicol. 14, 46 (2017).
Papadimitriou, C. et al. 3D culture method for Alzheimer’s disease modeling reveals interleukin-4 rescues Aβ42-induced loss of human neural stem cell plasticity. Dev. Cell 46, 85–101.e8 (2018).
Fang, Y. et al. The adhesion and migration of microglia to β-amyloid (Aβ) is decreased with aging and inhibited by Nogo/NgR pathway. J. Neuroinflammation 15, 210 (2018).
Omar Zaki, S. S., Kanesan, L., Leong, M. Y. D. & Vidyadaran, S. The influence of serum-supplemented culture media in a transwell migration assay. Cell Biol. Int. 43, 1201–1204 (2019).
Wang, S. et al. α-Synuclein, a chemoattractant, directs microglial migration via H2O2-dependent Lyn phosphorylation. Proc. Natl Acad. Sci. USA 112, E1926–E1935 (2015).
Wu, H.-j. et al. Analysis of microglial migration by a micropipette assay. Nat. Protoc. 9, 491–500 (2014).
Akther, F., Yakob, S. B., Nguyen, N. T. & Ta, H. T. Surface modification techniques for endothelial cell seeding in PDMS microfluidic devices. Biosensors 10, 182 (2020).
Fan, D.-H., Yuan, S.-W. & Shen, Y.-M. Surface modification with BSA blocking based on in situ synthesized gold nanoparticles in poly(dimethylsiloxane) microchip. Colloids Surf. B 75, 608–611 (2010).
Trantidou, T., Elani, Y., Parsons, E. & Ces, O. Hydrophilic surface modification of PDMS for droplet microfluidics using a simple, quick, and robust method via PVA deposition. Microsyst. Nanoeng. 3, 16091 (2017).
Wu, D., Zhao, B., Dai, Z., Qin, J. & Lin, B. Grafting epoxy-modified hydrophilic polymers onto poly(dimethylsiloxane) microfluidic chip to resist nonspecific protein adsorption. Lab Chip 6, 942–947 (2006).
Ren, K., Zhou, J. & Wu, H. Materials for microfluidic chip fabrication. Acc. Chem. Res. 46, 2396–2406 (2013).
Zhang, B., Korolj, A., Lai, B. F. L. & Radisic, M. Advances in organ-on-a-chip engineering. Nat. Rev. Mater. 3, 257–278 (2018).
Bhatia, S. N. & Ingber, D. E. Microfluidic organs-on-chips. Nat. Biotechnol. 32, 760–772 (2014).
Ralay Ranaivo, H. & Wainwright, M. S. Albumin activates astrocytes and microglia through mitogen-activated protein kinase pathways. Brain Res. 1313, 222–231 (2010).
Schafer, D. P. et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 74, 691–705 (2012).
Li, Y., Du, X.-F., Liu, C.-S., Wen, Z.-L. & Du, J.-L. Reciprocal regulation between resting microglial dynamics and neuronal activity in vivo. Dev. Cell 23, 1189–1202 (2012).
Alghamri, M. S. et al. G-CSF secreted by mutant IDH1 glioma stem cells abolishes myeloid cell immunosuppression and enhances the efficacy of immunotherapy. Sci. Adv. 7, eabh3243 (2021).
Sielska, M. et al. Tumour-derived CSF2/granulocyte macrophage colony stimulating factor controls myeloid cell accumulation and progression of gliomas. Br. J. Cancer 123, 438–448 (2020).
Ghoochani, A. et al. MIF-CD74 signaling impedes microglial M1 polarization and facilitates brain tumorigenesis. Oncogene 35, 6246–6261 (2016).
Yonai, R., Sato, R. & Nasu, M. Cerebral edema induced by hyperammonemia: a case report. Am. J. Emerg. Med. 34, 2461.e3–2461.e5 (2016).
Rama Rao, K. V., Jayakumar, A. R., Tong, X., Curtis, K. M. & Norenberg, M. D. Brain aquaporin-4 in experimental acute liver failure. J. Neuropathol. Exp. Neurol. 69, 869–879 (2010).
Rungta, R. L. et al. The cellular mechanisms of neuronal swelling underlying cytotoxic edema. Cell 161, 610–621 (2015).
Back, A. et al. Ammonia-induced brain swelling and neurotoxicity in an organotypic slice model. Neurol. Res. 33, 1100–1108 (2011).
Tanaka, S. et al. CD206 expression in induced microglia-like cells from peripheral blood as a surrogate biomarker for the specific immune microenvironment of neurosurgical diseases including glioma. Front. Immunol. 12, 670131 (2021).
Bodega, G. et al. Ammonia induces aquaporin-4 rearrangement in the plasma membrane of cultured astrocytes. Neurochem. Int. 61, 1314–1324 (2012).
Scott, T. R., Kronsten, V. T., Hughes, R. D. & Shawcross, D. L. Pathophysiology of cerebral oedema in acute liver failure. World J. Gastroenterol. 19, 9240–9255 (2013).
Angelova, P. R. et al. Hyperammonaemia induces mitochondrial dysfunction and neuronal cell death. JHEP Rep. 4, 100510 (2022).
Niknahad, H., Jamshidzadeh, A., Heidari, R., Zarei, M. & Ommati, M. M. Ammonia-induced mitochondrial dysfunction and energy metabolism disturbances in isolated brain and liver mitochondria, and the effect of taurine administration: relevance to hepatic encephalopathy treatment. Clin. Exp. Hepatol. 3, 141–151 (2017).
Acknowledgements
This work was supported by the National Research Foundation (NRF-2020R1A2C2010285, NRF-I21SS7606036), the Ministry of Health & Welfare and Ministry of Science and ICT (HU22C0115) through the Korea Health Industry Development Institute and Korea Dementia Research Center, Collabo R&D between Industry, Academy and Research Institutes funded by the Korea Ministry of SMEs and Startups in 2022 (no. S3247358) to H.C. and NRF-2022R1I1A1A01063094 to Y.J.K.
Author information
Authors and Affiliations
Contributions
H.C. supervised the whole project. Y.J.K. mainly performed the experiments and wrote the manuscript. Y.N.D., M.T., V.T.A.T., G.A. and H.N. contributed to performing the experiments and writing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Protocols thanks Caghan Kizil and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Cho, H. et al. Sci. Rep. 3, 1823 (2013): https://doi.org/10.1038/srep01823
Park, J. et al. Nat. Neurosci. 21, 941–951 (2018): https://doi.org/10.1038/s41593-018-0175-4
McQuade, A. et al. Nat. Commun. 11, 5370 (2020): https://doi.org/10.1038/s41467-020-19227-5
Chun, H. et al. Nat. Neurosci. 23, 1555–1566 (2020): https://doi.org/10.1038/s41593-020-00735-y
Kang, Y. J. et al. Adv. Sci. 8, 2101251 (2021): https://doi.org/10.1002/advs.202101251
Supplementary information
Supplementary Information
Supplementary Figs. 1–7.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kang, Y.J., Diep, Y.N., Tran, M. et al. Three-dimensional human neural culture on a chip recapitulating neuroinflammation and neurodegeneration. Nat Protoc 18, 2838–2867 (2023). https://doi.org/10.1038/s41596-023-00861-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-023-00861-4
This article is cited by
-
Modeling the neuroimmune system in Alzheimer’s and Parkinson’s diseases
Journal of Neuroinflammation (2024)
-
Environmental aluminum oxide inducing neurodegeneration in human neurovascular unit with immunity
Scientific Reports (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.