Abstract
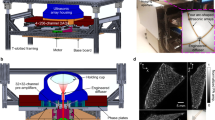
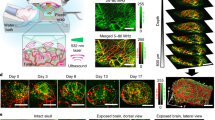
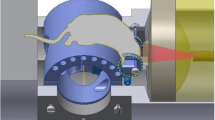
Fast tracking of biological dynamics across multiple murine organs using the currently commercially available whole-body preclinical imaging systems is hindered by their limited contrast, sensitivity and spatial or temporal resolution. Spiral volumetric optoacoustic tomography (SVOT) provides optical contrast, with an unprecedented level of spatial and temporal resolution, by rapidly scanning a mouse using spherical arrays, thus overcoming the current limitations in whole-body imaging. The method enables the visualization of deep-seated structures in living mammalian tissues in the near-infrared spectral window, while further providing unrivalled image quality and rich spectroscopic optical contrast. Here, we describe the detailed procedures for SVOT imaging of mice and provide specific details on how to implement a SVOT system, including component selection, system arrangement and alignment, as well as the image processing methods. The step-by-step guide for the rapid panoramic (360°) head-to-tail whole-body imaging of a mouse includes the rapid visualization of contrast agent perfusion and biodistribution. The isotropic spatial resolution possible with SVOT can reach 90 µm in 3D, while alternative steps enable whole-body scans in less than 2 s, unattainable with other preclinical imaging modalities. The method further allows the real-time (100 frames per second) imaging of biodynamics at the whole-organ level. The multiscale imaging capacity provided by SVOT can be used for visualizing rapid biodynamics, monitoring responses to treatments and stimuli, tracking perfusion, and quantifying total body accumulation and clearance dynamics of molecular agents and drugs. Depending on the imaging procedure, the protocol requires 1–2 h to complete by users trained in animal handling and biomedical imaging.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The statistical source data for Fig. 2c and Fig. 6f–h can be downloaded through the following link: https://doi.org/10.6084/m9.figshare.22257232. The raw source data for Fig. 4 can be downloaded through the following link: https://doi.org/10.6084/m9.figshare.21769340. The 3D computer aided design models of the custom-engineered animal can be downloaded through the following link: https://doi.org/10.6084/m9.figshare.21707867. All other raw datasets are available for research purposes from the corresponding author upon reasonable request. Source data are provided with this paper.
Code availability
The SVOT reconstruction code for Fig. 4a is publicly available through the following link: https://doi.org/10.6084/m9.figshare.21765299. The SVOT acquisition codes and reconstruction codes for the remaining figures are available from the corresponding author upon reasonable request.
References
Kiessling, F. & Pichler, B. J. Small Animal Imaging: Basics and Practical Guide (Springer Science & Business Media, 2010).
Baker, M. The whole picture. Nature 463, 977–979 (2010).
van der Heyden, B., Roden, S., Dok, R., Nuyts, S. & Sterpin, E. Virtual monoenergetic micro-CT imaging in mice with artificial intelligence. Sci. Rep. 12, 2324 (2022).
Shaker, K., Häggmark, I., Reichmann, J., Arsenian-Henriksson, M. & Hertz, H. M. Phase-contrast X-ray tomography resolves the terminal bronchioles in free-breathing mice. Commun. Phys. 4, 1–9 (2021).
Qin, R. et al. Carbonized paramagnetic complexes of Mn (II) as contrast agents for precise magnetic resonance imaging of sub-millimeter-sized orthotopic tumors. Nat. Commun. 13, 1938 (2022).
Zhan, S. et al. Targeting NQO1/GPX4-mediated ferroptosis by plumbagin suppresses in vitro and in vivo glioma growth. Br. J. Cancer 127, 1–13 (2022).
Kim, D.-Y. et al. In vivo imaging of invasive aspergillosis with 18F-fluorodeoxysorbitol positron emission tomography. Nat. Commun. 13, 1926 (2022).
Li, D. et al. SARS-CoV-2 receptor binding domain radio-probe: a non-invasive approach for angiotensin-converting enzyme 2 mapping in mice. Acta Pharmacol. Sin. 43, 1–9 (2021).
Zhang, Y. et al. Augmented ultrasonography with implanted CMOS electronic motes. Nat. Commun. 13, 3521 (2022).
Heiles, B. et al. Performance benchmarking of microbubble-localization algorithms for ultrasound localization microscopy. Nat. Biomed. Eng. 6, 605–616 (2022).
Enninful, A., Baysoy, A. & Fan, R. Unmixing for ultra-high-plex fluorescence imaging. Nat. Commun. 13, 3473 (2022).
Glaser, A. K. et al. A hybrid open-top light-sheet microscope for versatile multi-scale imaging of cleared tissues. Nat. Methods 19, 613–619 (2022).
Bouchard, M. B. et al. Swept confocally-aligned planar excitation (SCAPE) microscopy for high-speed volumetric imaging of behaving organisms. Nat. Photon. 9, 113–119 (2015).
Santos‐Coquillat, A. et al. Goat milk exosomes as natural nanoparticles for detecting inflammatory processes by optical imaging. Small 18, 2105421 (2022).
Beard, P. Biomedical photoacoustic imaging. Interface Focus 1, 602–631 (2011).
Manohar, S. & Razansky, D. Photoacoustics: a historical review. Adv. Opt. Photonics 8, 586–617 (2016).
Das, D., Sharma, A., Rajendran, P. & Pramanik, M. Another decade of photoacoustic imaging. Phys. Med. Biol. 66, 05TR01 (2021).
Deán-Ben, X. L., Gottschalk, S., Mc Larney, B., Shoham, S. & Razansky, D. Advanced optoacoustic methods for multiscale imaging of in vivo dynamics. Chem. Soc. Rev. 46, 2158–2198 (2017).
Deán‐Ben, X. L. & Razansky, D. Optoacoustic imaging of the skin. Exp. Dermatol. 30, 1598–1609 (2021).
Wang, L. V. & Hu, S. Photoacoustic tomography: in vivo imaging from organelles to organs. Science 335, 1458–1462 (2012).
Kruger, R. A., Kiser, W. L. Jr, Reinecke, D. R. & Kruger, G. A. Thermoacoustic computed tomography using a conventional linear transducer array. Med. Phys. 30, 856–860 (2003).
Wang, X. et al. Noninvasive laser-induced photoacoustic tomography for structural and functional in vivo imaging of the brain. Nat. Biotechnol. 21, 803–806 (2003).
Xia, J. & Wang, L. V. Small-animal whole-body photoacoustic romography: a review. IEEE Trans. Biomed. Eng. 61, 1380–1389 (2014).
Jeon, M., Kim, J. & Kim, C. Multiplane spectroscopic whole-body photoacoustic imaging of small animals in vivo. Med. Biol. Eng. Comput. 54, 283–294 (2016).
Ma, R., Taruttis, A., Ntziachristos, V. & Razansky, D. Multispectral optoacoustic tomography (MSOT) scanner for whole-body small animal imaging. Opt. Express 17, 21414–21426 (2009).
Gateau, J., Caballero, M. Á. A., Dima, A. & Ntziachristos, V. Three‐dimensional optoacoustic tomography using a conventional ultrasound linear detector array: whole‐body tomographic system for small animals. Med. Phys. 40, 013302 (2013).
Brecht, H.-P. F. et al. Whole-body three-dimensional optoacoustic tomography system for small animals. J. Biomed. Opt. 14, 064007 (2009).
Xia, J. et al. Whole-body ring-shaped confocal photoacoustic computed tomography of small animals in vivo. J. Biomed. Opt. 17, 050506 (2012).
Razansky, D., Buehler, A. & Ntziachristos, V. Volumetric real-time multispectral optoacoustic tomography of biomarkers. Nat. Protoc. 6, 1121–1129 (2011).
Lv, J. et al. Hemispherical photoacoustic imaging of myocardial infarction: in vivo detection and monitoring. Eur. Radiol. 28, 2176–2183 (2018).
Gottschalk, S. et al. Rapid volumetric optoacoustic imaging of neural dynamics across the mouse brain. Nat. Biomed. Eng. 3, 392–401 (2019).
Gottschalk, S., Felix Fehm, T., Luís Deán-Ben, X. & Razansky, D. Noninvasive real-time visualization of multiple cerebral hemodynamic parameters in whole mouse brains using five-dimensional optoacoustic tomography. J. Cerebr. Blood Flow. Metab. 35, 531–535 (2015).
Fehm, T. F., Deán-Ben, X. L., Ford, S. J. & Razansky, D. In vivo whole-body optoacoustic scanner with real-time volumetric imaging capacity. Optica 3, 1153–1159 (2016).
Deán-Ben, X. L., Fehm, T. F., Ford, S. J., Gottschalk, S. & Razansky, D. Spiral volumetric optoacoustic tomography visualizes multi-scale dynamics in mice. Light Sci. Appl. 6, e16247 (2017).
Ron, A., Kalva, S. K., Periyasamy, V., Deán‐Ben, X. L. & Razansky, D. Flash scanning volumetric optoacoustic tomography for high resolution whole‐body tracking of nanoagent kinetics and biodistribution. Laser Photonics Rev. 15, 2000484 (2021).
Kalva, S. K., Dean-Ben, X. L. & Razansky, D. Single-sweep volumetric optoacoustic tomography of whole mice. Photonics Res. 9, 899–908 (2021).
Kalva, S. K., Sánchez-Iglesias, A., Deán-Ben, X. L., Liz-Marzán, L. M. & Razansky, D. Rapid volumetric optoacoustic tracking of nanoparticle kinetics across murine organs. ACS Appl. Mater. Interfaces 4, 172–178 (2021).
Ron, A., Deán-Ben, X. L., Gottschalk, S. & Razansky, D. Volumetric optoacoustic imaging unveils high-resolution patterns of acute and cyclic hypoxia in a murine model of breast cancer. Cancer Res. 79, 4767–4775 (2019).
Ivankovic, I. et al. Volumetric optoacoustic tomography enables non-invasive in vivo characterization of impaired heart function in hypoxic conditions. Sci. Rep. 9, 8369 (2019).
Berghen, N. et al. Radiosafe micro-computed tomography for longitudinal evaluation of murine disease models. Sci. Rep. 9, 17598 (2019).
Chen, X. et al. Mapping optogenetically-driven single-vessel fMRI with concurrent neuronal calcium recordings in the rat hippocampus. Nat. Commun. 10, 5239 (2019).
Zhang, P. et al. A review of advances in imaging methodology in fluorescence molecular tomography. Phys. Med. Biol. 67, 10TR01 (2022).
Ntziachristos, V., Ripoll, J., Wang, L. V. & Weissleder, R. Looking and listening to light: the evolution of whole-body photonic imaging. Nat. Biotechnol. 23, 313–320 (2005).
Patwardhan, S. V., Bloch, S. R., Achilefu, S. & Culver, J. P. Time-dependent whole-body fluorescence tomography of probe bio-distributions in mice. Opt. Express 13, 2564–2577 (2005).
Hu, P., Li, L., Lin, L. & Wang, L. V. Spatiotemporal antialiasing in photoacoustic computed tomography. IEEE Trans. Med. Imaging 39, 3535–3547 (2020).
Rockwell, B., Thomas, R. & Zimmerman, S. in International Laser Safety Conference. 75–77 (Laser Institute of America, 2015).
Dean-Ben, X. L. & Razansky, D. Portable spherical array probe for volumetric real-time optoacoustic imaging at centimeter-scale depths. Opt. Express 21, 28062–28071 (2013).
Dean-Ben, X. L., Ozbek, A. & Razansky, D. Volumetric real-time tracking of peripheral human vasculature with GPU-accelerated three-dimensional optoacoustic tomography. IEEE Trans. Med. Imaging 32, 2050–2055 (2013).
Deán-Ben, X. L., Özbek, A. & Razansky, D. Accounting for speed of sound variations in volumetric hand-held optoacoustic imaging. Front. Optoelectron. 10, 280–286 (2017).
Szabo, T. L. Diagnostic Ultrasound Imaging: Inside Out (Academic Press, 2004).
Ron, A., Davoudi, N., Deán-Ben, X. L. & Razansky, D. Self-gated respiratory motion rejection for optoacoustic tomography. Appl. Sci. 9, 2737 (2019).
Dean-Ben, X. L., Ford, S. J. & Razansky, D. High-frame rate four dimensional optoacoustic tomography enables visualization of cardiovascular dynamics and mouse heart perfusion. Sci. Rep. 5, 10133 (2015).
Merčep, E., Burton, N. C., Claussen, J. & Razansky, D. Whole-body live mouse imaging by hybrid reflection-mode ultrasound and optoacoustic tomography. Opt. Lett. 40, 4643–4646 (2015).
Lutzweiler, C. & Razansky, D. Optoacoustic imaging and tomography: reconstruction approaches and outstanding challenges in image performance and quantification. Sensors 13, 7345–7384 (2013).
Chen, Z. et al. Simultaneous functional magnetic resonance and optoacoustic imaging of brain‐wide sensory responses in mice. Adv. Sci. 10, e2205191 (2022).
Lin, H.-C. A. et al. Characterization of cardiac dynamics in an acute myocardial infarction model by four-dimensional optoacoustic and magnetic resonance imaging. Theranostics 7, 4470 (2017).
Acknowledgements
The authors acknowledge support from the Swiss National Science Foundation (grant 310030_192757) and the European Research Council (grant ERC-2015-CoG-682379).
Author information
Authors and Affiliations
Contributions
S.K.K., X.L.D.-B. and D.R. designed the imaging system and experiments. S.K.K. performed the experiments and analyzed the data. X.L.D.-B. provided reconstruction algorithms and assisted in data analysis. M.R. assisted in animal preparation and handling. D.R. supervised the study. All authors contributed to writing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Protocols thanks Miya Ishihara, Yash Mantri and Kanyi Pu for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Kalva, S. K. et al. ACS Appl. Mater. Interfaces 14, 172–178 (2022): https://doi.org/10.1021/acsami.1c17661
Ron, A. et al. Laser Phot. Rev. 15, 2000484 (2021): https://doi.org/10.1002/lpor.202000484
Deán-Ben, X. L. et al. Light Sci. Appl. 6, e16247 (2017): https://doi.org/10.1038/lsa.2016.247
Supplementary information
Supplementary Information
Supplementary Figs. 1–3.
Source data
Source Data Fig. 2
Statistical source data.
Source Data Fig. 6
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kalva, S.K., Deán-Ben, X.L., Reiss, M. et al. Spiral volumetric optoacoustic tomography for imaging whole-body biodynamics in small animals. Nat Protoc 18, 2124–2142 (2023). https://doi.org/10.1038/s41596-023-00834-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-023-00834-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.