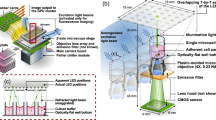
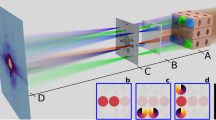
Abstract
First envisioned for determining crystalline structures, ptychography has become a useful imaging tool for microscopists. However, ptychography remains underused by biomedical researchers due to its limited resolution and throughput in the visible light regime. Recent developments of spatial- and Fourier-domain ptychography have successfully addressed these issues and now offer the potential for high-resolution, high-throughput optical imaging with minimal hardware modifications to existing microscopy setups, often providing an excellent trade-off between resolution and field of view inherent to conventional imaging systems, giving biomedical researchers the best of both worlds. Here, we provide extensive information to enable the implementation of ptychography by biomedical researchers in the visible light regime. We first discuss the intrinsic connections between spatial-domain coded ptychography and Fourier ptychography. A step-by-step guide then provides the user instructions for developing both systems with practical examples. In the spatial-domain implementation, we explain how a large-scale, high-performance blood-cell lens can be made at negligible expense. In the Fourier-domain implementation, we explain how adding a low-cost light source to a regular microscope can improve the resolution beyond the limit of the objective lens. The turnkey operation of these setups is suitable for use by professional research laboratories, as well as citizen scientists. Users with basic experience in optics and programming can build the setups within a week. The do-it-yourself nature of the setups also allows these procedures to be implemented in laboratory courses related to Fourier optics, biomedical instrumentation, digital image processing, robotics and capstone projects.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

















Similar content being viewed by others
Data availability
The main data supporting this study are available within the article, Supplementary Data and the primary supporting study10,11,14. Experimental datasets for both setups in this study are available in Zenodo: https://doi.org/10.5281/zenodo.7492626.
Code availability
All related MATLAB and Arduino code is provided in Supplementary Software. Additional code for testing experimental datasets is available in Zenodo: https://doi.org/10.5281/zenodo.7492626.
References
Sayre, D. Some implications of a theorem due to Shannon. Acta Crystallogr. 5, 843–843 (1952).
Hoppe, W. Diffraction in inhomogeneous primary wave fields. 1. Principle of phase determination from electron diffraction interference. Acta Crystallogr 25, 495–501 (1969).
Guizar-Sicairos, M. & Thibault, P. Ptychography: a solution to the phase problem. Phys. Today 74, 42–48 (2021).
Faulkner, H. M. L. & Rodenburg, J. Movable aperture lensless transmission microscopy: a novel phase retrieval algorithm. Phys. Rev. Lett. 93, 023903 (2004).
Rodenburg, J. & Maiden, A. in Springer Handbook of Microscopy 819–904 (Springer, 2019).
Wang, T. et al. Optical ptychography for biomedical imaging: recent progress and future directions. Biomed. Opt. Express 14, 489–532 (2023).
Loetgering, L., Witte, S. & Rothhardt, J. Advances in laboratory-scale ptychography using high harmonic sources. Opt. Express 30, 4133–4164 (2022).
Pfeiffer, F. X-ray ptychography. Nat. Photonics 12, 9–17 (2018).
Jiang, Y. et al. Electron ptychography of 2D materials to deep sub-ångström resolution. Nature 559, 343–349 (2018).
Zheng, G., Horstmeyer, R. & Yang, C. Wide-field, high-resolution Fourier ptychographic microscopy. Nat. Photonics 7, 739 (2013).
Jiang, S. et al. Resolution-enhanced parallel coded ptychography for high-throughput optical imaging. ACS Photonics 8, 3261–3271 (2021).
Jiang, S. et al. High-throughput digital pathology via a handheld, multiplexed, and AI-powered ptychographic whole slide scanner. Lab Chip 22, 2657–2670 (2022).
Jiang, S. et al. Blood-coated sensor for high-throughput ptychographic cytometry on a Blu-ray disc. ACS Sens. 7, 1058–1067 (2022).
Jiang, S. et al. Ptychographic sensor for large-scale lensless microbial monitoring with high spatiotemporal resolution. Biosens. Bioelectron. 196, 113699 (2022).
Zheng, G., Shen, C., Jiang, S., Song, P. & Yang, C. Concept, implementations and applications of Fourier ptychography. Nat. Rev. Phys. 3, 207–223 (2021).
Pan, A., Zuo, C. & Yao, B. High-resolution and large field-of-view Fourier ptychographic microscopy and its applications in biomedicine. Rep. Prog. Phys. 83, 096101 (2020).
Konda, P. C. et al. Fourier ptychography: current applications and future promises. Opt. Express 28, 9603–9630 (2020).
Guo, K., Dong, S. & Zheng, G. Fourier ptychography for brightfield, phase, darkfield, reflective, multi-slice, and fluorescence imaging. IEEE J. Sel. Top. Quantum Electron. 22, 77–88 (2015).
Horstmeyer, R., Chung, J., Ou, X., Zheng, G. & Yang, C. Diffraction tomography with Fourier ptychography. Optica 3, 827–835 (2016).
Zuo, C., Sun, J., Li, J., Asundi, A. & Chen, Q. Wide-field high-resolution 3D microscopy with Fourier ptychographic diffraction tomography. Opt. Lasers Eng. 128, 106003 (2020).
Dong, S. et al. Aperture-scanning Fourier ptychography for 3D refocusing and super-resolution macroscopic imaging. Opt. Express 22, 13586–13599 (2014).
Holloway, J., Wu, Y., Sharma, M. K., Cossairt, O. & Veeraraghavan, A. SAVI: synthetic apertures for long-range, subdiffraction-limited visible imaging using Fourier ptychography. Sci. Adv. 3, e1602564 (2017).
Zhang, H. et al. Near-field Fourier ptychography: super-resolution phase retrieval via speckle illumination. Opt. Express 27, 7498–7512 (2019).
Xiang, M. et al. Coherent synthetic aperture imaging for visible remote sensing via reflective Fourier ptychography. Opt. Lett. 46, 29–32 (2021).
Wakonig, K. et al. X-ray Fourier ptychography. Sci. Adv. 5, eaav0282 (2019).
Zhang, F., Pedrini, G. & Osten, W. Phase retrieval of arbitrary complex-valued fields through aperture-plane modulation. Phys. Rev. A 75, 043805 (2007).
Maiden, A. M., Rodenburg, J. M. & Humphry, M. J. Optical ptychography: a practical implementation with useful resolution. Opt. Lett. 35, 2585–2587 (2010).
Song, P. et al. Super-resolution microscopy via ptychographic structured modulation of a diffuser. Opt. Lett. 44, 3645–3648 (2019).
Thibault, P. & Menzel, A. Reconstructing state mixtures from diffraction measurements. Nature 494, 68–71 (2013).
Batey, D. J., Claus, D. & Rodenburg, J. M. Information multiplexing in ptychography. Ultramicroscopy 138, 13–21 (2014).
Song, P. et al. Super-resolved multispectral lensless microscopy via angle-tilted, wavelength-multiplexed ptychographic modulation. Opt. Lett. 45, 3486–3489 (2020).
Thibault, P., Dierolf, M., Bunk, O., Menzel, A. & Pfeiffer, F. Probe retrieval in ptychographic coherent diffractive imaging. Ultramicroscopy 109, 338–343 (2009).
Guizar-Sicairos, M. & Fienup, J. R. Phase retrieval with transverse translation diversity: a nonlinear optimization approach. Opt. Express 16, 7264–7278 (2008).
Maiden, A. M. & Rodenburg, J. M. An improved ptychographical phase retrieval algorithm for diffractive imaging. Ultramicroscopy 109, 1256–1262 (2009).
Ou, X., Zheng, G. & Yang, C. Embedded pupil function recovery for Fourier ptychographic microscopy. Opt. Express 22, 4960–4972 (2014).
Song, P. et al. Full-field Fourier ptychography (FFP): spatially varying pupil modeling and its application for rapid field-dependent aberration metrology. APL Photonics 4, 050802 (2019).
Gustafsson, M. G. Surpassing the lateral resolution limit by a factor of two using structured illumination microscopy. J. Microsc. 198, 82–87 (2000).
Liang, M. & Yang, C. Implementation of free-space Fourier ptychography with near maximum system numerical aperture. Opt. Express 30, 20321–20332 (2022).
Schiske, P. Image reconstruction by means of focus series. In Proc. 4th European Conference on Electron Microscopy, Rome, Italy (Tipografia poliglotta, 1968).
Bao, P., Zhang, F., Pedrini, G. & Osten, W. Phase retrieval using multiple illumination wavelengths. Opt. Lett. 33, 309–311 (2008).
Greenbaum, A. & Ozcan, A. Maskless imaging of dense samples using pixel super-resolution based multi-height lensfree on-chip microscopy. Opt. Express 20, 3129–3143 (2012).
Luo, W., Zhang, Y., Feizi, A., Göröcs, Z. & Ozcan, A. Pixel super-resolution using wavelength scanning. Light Sci. Appl. 5, e16060–e16060 (2016).
Zuo, C. et al. Transport of intensity equation: a tutorial. Opt. Lasers Eng. 135, 106187 (2020).
Fienup, J. R. Phase retrieval algorithms: a comparison. Appl. Opt. 21, 2758–2769 (1982).
Bishara, W., Su, T.-W., Coskun, A. F. & Ozcan, A. Lensfree on-chip microscopy over a wide field-of-view using pixel super-resolution. Opt. Express 18, 11181–11191 (2010).
Xu, W., Jericho, M., Meinertzhagen, I. & Kreuzer, H. Digital in-line holography for biological applications. Proc. Natl Acad. Sci. USA 98, 11301–11305 (2001).
Guo, C. et al. Quantitative multi-height phase retrieval via a coded image sensor. Biomed. Opt. Express 12, 7173–7184 (2021).
Abels, E. & Pantanowitz, L. Current state of the regulatory trajectory for whole slide imaging devices in the USA. J. Pathol. Inform. 8, 23 (2017).
Bian, Z. et al. Autofocusing technologies for whole slide imaging and automated microscopy. J. Biophotonics 13, e202000227 (2020).
Wang, T. et al. Remote referencing strategy for high-resolution coded ptychographic imaging. Opt. Lett. 48, 485–488 (2023).
Chan, A. C. et al. Parallel Fourier ptychographic microscopy for high-throughput screening with 96 cameras (96 eyes). Sci. Rep. 9, 1–12 (2019).
Wakefield, D. L. et al. Cellular analysis using label-free parallel array microscopy with Fourier ptychography. Biomed. Opt. Express 13, 1312–1327 (2022).
Li, P. & Maiden, A. Lensless LED matrix ptychographic microscope: problems and solutions. Appl. Opt. 57, 1800–1806 (2018).
Shu, Y. et al. Adaptive optical quantitative phase imaging based on annular illumination Fourier ptychographic microscopy. PhotoniX 3, 1–15 (2022).
Horstmeyer, R., Ou, X., Zheng, G., Willems, P. & Yang, C. Digital pathology with Fourier ptychography. Comput. Med. Imaging Graph. 42, 38–43 (2015).
Williams, A. J. et al. Fourier ptychographic microscopy for filtration-based circulating tumor cell enumeration and analysis. J. Biomed. Opt. 19, 066007 (2014).
Chen, J. et al. Rapid full-color Fourier ptychographic microscopy via spatially filtered color transfer. Photonics Res. 10, 2410–2421 (2022).
Ou, X., Horstmeyer, R., Yang, C. & Zheng, G. Quantitative phase imaging via Fourier ptychographic microscopy. Opt. Lett. 38, 4845–4848 (2013).
Jiang, S. et al. Wide-field, high-resolution lensless on-chip microscopy via near-field blind ptychographic modulation. Lab Chip 20, 1058–1065 (2020).
Sun, J., Zuo, C., Zhang, J., Fan, Y. & Chen, Q. High-speed Fourier ptychographic microscopy based on programmable annular illuminations. Sci. Rep. 8, 1–12 (2018).
Tian, L. et al. Computational illumination for high-speed in vitro Fourier ptychographic microscopy. Optica 2, 904–911 (2015).
Chowdhury, S. et al. High-resolution 3D refractive index microscopy of multiple-scattering samples from intensity images. Optica 6, 1211–1219 (2019).
Guo, C. et al. Depth-multiplexed ptychographic microscopy for high-throughput imaging of stacked bio-specimens on a chip. Biosens. Bioelectron. 224, 115049 (2023).
Chung, J., Ou, X., Kulkarni, R. P. & Yang, C. Counting white blood cells from a blood smear using Fourier ptychographic microscopy. PloS ONE 10, e0133489 (2015).
Song, P. et al. Optofluidic ptychography on a chip. Lab Chip 21, 4549–4556 (2021).
Maiden, A. M., Humphry, M. J. & Rodenburg, J. Ptychographic transmission microscopy in three dimensions using a multi-slice approach. J. Opt. Soc. Am. A 29, 1606–1614 (2012).
Dong, S., Nanda, P., Shiradkar, R., Guo, K. & Zheng, G. High-resolution fluorescence imaging via pattern-illuminated Fourier ptychography. Opt. Express 22, 20856–20870 (2014).
Guo, K. et al. 13-fold resolution gain through turbid layer via translated unknown speckle illumination. Biomed. Opt. Express 9, 260–275 (2018).
Yeh, L.-H., Chowdhury, S. & Waller, L. Computational structured illumination for high-content fluorescence and phase microscopy. Biomed. Opt. Express 10, 1978–1998 (2019).
Dong, S., Nanda, P., Guo, K., Liao, J. & Zheng, G. Incoherent Fourier ptychographic photography using structured light. Photonics Res. 3, 19–23 (2015).
Ou, X., Horstmeyer, R., Zheng, G. & Yang, C. High numerical aperture Fourier ptychography: principle, implementation and characterization. Opt. Express 23, 3472–3491 (2015).
Sun, J., Zuo, C., Zhang, L. & Chen, Q. Resolution-enhanced Fourier ptychographic microscopy based on high-numerical-aperture illuminations. Sci. Rep. 7, 1–11 (2017).
Zheng, G. Fourier Ptychographic Imaging: A Matlab Tutorial (Morgan & Claypool Publishers, 2016).
Phillips, Z. F., Eckert, R. & Waller, L. Quasi-dome: a self-calibrated high-NA LED illuminator for Fourier ptychography. In Imaging Systems and Applications (Optica Publishing Group, 2017).
Pan, A. et al. Subwavelength resolution Fourier ptychography with hemispherical digital condensers. Opt. Express 26, 23119–23131 (2018).
Guo, K., Dong, S., Nanda, P. & Zheng, G. Optimization of sampling pattern and the design of Fourier ptychographic illuminator. Opt. Express 23, 6171–6180 (2015).
Yeh, L.-H. et al. Experimental robustness of Fourier ptychography phase retrieval algorithms. Opt. Express 23, 33214–33240 (2015).
Maiden, A., Johnson, D. & Li, P. Further improvements to the ptychographical iterative engine. Optica 4, 736–745 (2017).
Bian, Z., Dong, S. & Zheng, G. Adaptive system correction for robust Fourier ptychographic imaging. Opt. Express 21, 32400–32410 (2013).
Yang, L., Liu, Z., Zheng, G. & Chang, H. Batch-based alternating direction methods of multipliers for Fourier ptychography. Opt. Express 30, 34750–34764 (2022).
Dong, S., Shiradkar, R., Nanda, P. & Zheng, G. Spectral multiplexing and coherent-state decomposition in Fourier ptychographic imaging. Biomed. Opt. Express 5, 1757–1767 (2014).
Song, P. et al. Synthetic aperture ptychography: coded sensor translation for joint spatial-Fourier bandwidth expansion. Photonics Res. 10, 1624–1632 (2022).
Guizar-Sicairos, M., Thurman, S. T. & Fienup, J. R. Efficient subpixel image registration algorithms. Opt. Lett. 33, 156–158 (2008).
Bian, L. et al. Content adaptive illumination for Fourier ptychography. Opt. Lett. 39, 6648–6651 (2014).
Tian, L., Li, X., Ramchandran, K. & Waller, L. Multiplexed coded illumination for Fourier Ptychography with an LED array microscope. Biomed. Opt. Express 5, 2376–2389 (2014).
Fan, Y. et al. Efficient synthetic aperture for phaseless Fourier ptychographic microscopy with hybrid coherent and incoherent illumination. Laser Photonics Rev. 17, 2200201 (2023).
Dong, S., Bian, Z., Shiradkar, R. & Zheng, G. Sparsely sampled Fourier ptychography. Opt. Express 22, 5455–5464 (2014).
Song, P. et al. Freeform illuminator for computational microscopy. Intell. Comput. 2, 0015 (2023).
Acknowledgements
We thank Z. Bian and A. Pirhanov for their assistance in sample preparation. This work was partially supported by the UConn SPARK grant, UConn Research Excellence Program, National Science Foundation award 2012140 and National Institute of Health award U01-NS113873. P.S. also acknowledges the support of the Thermo Fisher Scientific Fellowship.
Author information
Authors and Affiliations
Contributions
G.Z. conceived the project. S.J., P.S. and G.Z. designed the pipeline. S.J., P.S., T.W. and G.Z. developed the prototype systems and prepared the display items. S.J., P.S., T.W. and L.Y. developed the data acquisition and processing pipelines for the protocol. T.W. and C.G. prepared all SolidWorks design files for the protocols. All authors contributed to the writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
G.Z. is a named inventor on the following patents related to Fourier ptychography (US Patent, nos. 9,817,224, 9,864,184, 9,497,379) and coded ptychography (US Patent, no. 11,487,099).
Peer review
Peer review information
Nature Protocols thanks Zhengjun Liu, Fucai Zhang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Zheng, G. et al. Nat. Photonics 7, 739-745 (2013): https://doi.org/10.1038/nphoton.2013.187
Jiang, S. et al. ACS Photonics 8, 3261-3271 (2021): https://doi.org/10.1021/acsphotonics.1c01085
Jiang, S. et al. Biosens. Bioelectron. 196, 113699 (2022): https://doi.org/10.1016/j.bios.2021.113699
Supplementary information
Supplementary Information
Supplementary Figs. 1–4.
Supplementary Software 1
MATLAB code and Arduino code for FP and CP.
Supplementary Data 1
SolidWorks design files for FP and CP.
Supplementary Video 1
Operation of the FP platform.
Supplementary Video 2
Operation of the CP platform.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jiang, S., Song, P., Wang, T. et al. Spatial- and Fourier-domain ptychography for high-throughput bio-imaging. Nat Protoc 18, 2051–2083 (2023). https://doi.org/10.1038/s41596-023-00829-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-023-00829-4
This article is cited by
-
On the use of deep learning for phase recovery
Light: Science & Applications (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.