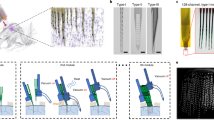
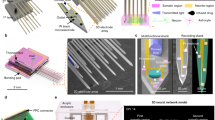
Abstract
Ultraflexible microelectrode arrays (MEAs) that can stably record from a large number of neurons after their chronic implantation offer opportunities for understanding neural circuit mechanisms and developing next-generation brain–computer interfaces. The implementation of ultraflexible MEAs requires their reliable implantation into deep brain tissues in a minimally invasive manner, as well as their precise integration with optogenetic tools to enable the simultaneous recording of neural activity and neuromodulation. Here, we describe the process for the preparation of elastocapillary self-assembled ultraflexible MEAs, their use in combination with adeno-associated virus vectors carrying opsin genes and promoters to form an optrode probe and their in vivo experimental use in the brains of rodents, enabling electrophysiological recordings and optical modulation of neuronal activity over long periods of time (on the order of weeks to months). The procedures, including device fabrication, probe assembly and implantation, can be completed within 3 weeks. The protocol is intended to facilitate the applications of ultraflexible MEAs for long-term neuronal activity recording and combined electrophysiology and optogenetics. The protocol requires users with expertise in clean room facilities for the fabrication of ultraflexible MEAs.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout













Similar content being viewed by others
Data availability
The raw recording data used to generate Fig. 13b–d have been deposited on Figshare (https://doi.org/10.6084/m9.figshare.c.6393534.v1). Source data are provided with this paper.
References
Buzsaki, G. Large-scale recording of neuronal ensembles. Nat. Neurosci. 7, 446–451 (2004).
Jun, J. J. et al. Fully integrated silicon probes for high-density recording of neural activity. Nature 551, 232–236 (2017).
Kollo, M. et al. CHIME: CMOS-hosted in vivo microelectrodes for massively scalable neuronal recordings. Front. Neurosci. 14, 834 (2020).
Obaid, A. et al. Massively parallel microwire arrays integrated with CMOS chips for neural recording. Sci. Adv. 6, eaay2789 (2020).
Gilletti, A. & Muthuswamy, J. Brain micromotion around implants in the rodent somatosensory cortex. J. Neural Eng. 3, 189–195 (2006).
Biran, R., Martin, D. C. & Tresco, P. A. Neuronal cell loss accompanies the brain tissue response to chronically implanted silicon microelectrode arrays. Exp. Neurol. 195, 115–126 (2005).
Polikov, V. S., Tresco, P. A. & Reichert, W. M. Response of brain tissue to chronically implanted neural electrodes. J. Neurosci. Methods 148, 1–18 (2005).
Williams, J. C., Rennaker, R. L. & Kipke, D. R. Long-term neural recording characteristics of wire microelectrode arrays implanted in cerebral cortex. Brain Res. Protoc. 4, 303–313 (1999).
Kozai, T. D. Y. et al. Chronic tissue response to carboxymethyl cellulose based dissolvable insertion needle for ultra-small neural probes. Biomaterials 35, 9255–9268 (2014).
Khodagholy, D. et al. Highly conformable conducting polymer electrodes for in vivo recordings. Adv. Mater. 23, H268–H272 (2011).
Tian, B. et al. Macroporous nanowire nanoelectronic scaffolds for synthetic tissues. Nat. Mater. 11, 986–994 (2012).
Kim, T.-i et al. Injectable, cellular-scale optoelectronics with applications for wireless optogenetics. Science 340, 211–216 (2013).
Liu, J. et al. Syringe-injectable electronics. Nat. Nanotechnol. 10, 629–636 (2015).
Someya, T., Bao, Z. & Malliaras, G. G. The rise of plastic bioelectronics. Nature 540, 379–385 (2016).
Luan, L. et al. Ultraflexible nanoelectronic probes form reliable, glial scar-free neural integration. Sci. Adv. 3, e1601966 (2017).
Park, S. et al. One-step optogenetics with multifunctional flexible polymer fibers. Nat. Neurosci. 20, 612–619 (2017).
Chung, J. E. et al. High-density, long-lasting, and multi-region electrophysiological recordings using polymer electrode arrays. Neuron 101, 21–31 (2019).
Guan, S. et al. Elastocapillary self-assembled neurotassels for stable neural activity recordings. Sci. Adv. 5, eaav2842 (2019).
Musk, E. An integrated brain-machine interface platform with thousands of channels. J. Med. Internet Res. 21, e16194 (2019).
Yang, X. et al. Bioinspired neuron-like electronics. Nat. Mater. 18, 510–517 (2019).
Fu, T.-M. et al. Stable long-term chronic brain mapping at the single-neuron level. Nat. Methods 13, 875–882 (2016).
Zhang, F. et al. Optogenetic interrogation of neural circuits: technology for probing mammalian brain structures. Nat. Protoc. 5, 439–456 (2010).
Anikeeva, P. et al. Optetrode: a multichannel readout for optogenetic control in freely moving mice. Nat. Neurosci. 15, 163–170 (2011).
Buzsáki, G. et al. Tools for probing local circuits: high-density silicon probes combined with optogenetics. Neuron 86, 92–105 (2015).
Zou, L. et al. Self-assembled multifunctional neural probes for precise integration of optogenetics and electrophysiology. Nat. Commun. 12, 5871 (2021).
McCall, J. G. et al. Fabrication and application of flexible, multimodal light-emitting devices for wireless optogenetics. Nat. Protoc. 8, 2413–2428 (2013).
McCall, J. G. et al. Preparation and implementation of optofluidic neural probes for in vivo wireless pharmacology and optogenetics. Nat. Protoc. 12, 219–237 (2017).
Gao, L. et al. Free-standing nanofilm electrode arrays for long-term stable neural interfacings. Adv. Mater. 34, e2107343 (2022).
Kozai, T. D. Y. & Kipke, D. R. Insertion shuttle with carboxyl terminated self-assembled monolayer coatings for implanting flexible polymer neural probes in the brain. J. Neurosci. Methods 184, 199–205 (2009).
Zhao, Z. et al. Nanoelectronic coating enabled versatile multifunctional neural probes. Nano Lett. 17, 4588–4595 (2017).
Wu, F. et al. Monolithically integrated μLEDs on silicon neural probes for high-resolution optogenetic studies in behaving animals. Neuron 88, 1136–1148 (2015).
Watakabe, A. et al. Comparative analyses of adeno-associated viral vector serotypes 1, 2, 5, 8 and 9 in marmoset, mouse and macaque cerebral cortex. Neurosci. Res. 93, 144–157 (2015).
Bennett, A. et al. Thermal stability as a determinant of AAV serotype identity. Mol. Ther. Methods Clin. Dev. 6, 171–182 (2017).
Deisseroth, K. Optogenetics: 10 years of microbial opsins in neuroscience. Nat. Neurosci. 18, 1213–1225 (2015).
Owen, S. F., Max, H. L. & Anatol, C. K. Thermal constraints on in vivo optogenetic manipulations. Nat. Neurosci. 22, 1061–1065 (2019).
Yang, Y. et al. Preparation and use of wireless reprogrammable multilateral optogenetic devices for behavioral neuroscience. Nat. Protoc. 17, 1073–1096 (2022).
van Asperen, J. V. et al. Determining glioma cell invasion and proliferation in ex vivo organotypic mouse brain slices using whole-mount immunostaining and tissue clearing. STAR Protoc. 3, 101703 (2022).
Acknowledgements
We thank the Fabrication Lab at NCNST for the microfabrication facilities and support and the Animal Resource Center at NCNST for animal housing and care. This work is supported by the National Natural Science Foundation of China (Grant Nos. 21790393, 61971150 and 32061143013) and the National Key Research and Development Program of China (Grant No. 2021ZD0202200).
Author information
Authors and Affiliations
Contributions
Y.F., H.T. and S.G. conceived the project and designed the detailed experimental protocol. S.G., J.D. and J.W. fabricated and characterized the devices. S.G., Y.Y. and M.L. performed animal surgery, electrophysiological recordings and corresponding analysis. Y.F., H.T. and S.G. wrote the paper. Y.F. acquired funding and supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Protocols thanks Shadi A. Dayeh and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Guan, S. et al. Sci. Adv. 5, eaav2842 (2019): https://doi.org/10.1126/sciadv.aav2842
Zou, L. et al. Nat. Commun. 12, 5871 (2021): https://doi.org/10.1038/s41467-021-26168-0
Gao, L. et al. Adv. Mater. 34, e2107343 (2022): https://doi.org/10.1002/adma.202107343
Supplementary information
Supplementary Data 1
Eight-filament ultraflexible microelectrode arrays
Supplementary Data 2
16-filament ultraflexible microelectrode arrays
Supplementary Data 3
120-pin FPC
Supplementary Data 4
120-pin PCB
Supplementary Data 5
3D-printed MEA holder
Supplementary Data 6
3D-printed optical fiber holder
Supplementary Data 7
3D-printed optrode cover
Supplementary Data 8
Stainless steel head plate
Source data
Source Data Fig. 13
Stastical data for Fig. 13, e–g
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guan, S., Tian, H., Yang, Y. et al. Self-assembled ultraflexible probes for long-term neural recordings and neuromodulation. Nat Protoc 18, 1712–1744 (2023). https://doi.org/10.1038/s41596-023-00824-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-023-00824-9
This article is cited by
-
A perspective on neuroethology: what the past teaches us about the future of neuroethology
Journal of Comparative Physiology A (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.