Abstract
Penetrating flexible electrode arrays can simultaneously record thousands of individual neurons in the brains of live animals. However, it has been challenging to spatially map and longitudinally monitor the dynamics of large three-dimensional neural networks. Here we show that optimized ultraflexible electrode arrays distributed across multiple cortical regions in head-fixed mice and in freely moving rats allow for months-long stable electrophysiological recording of several thousand neurons at densities of about 1,000 neural units per cubic millimetre. The chronic recordings enhanced decoding accuracy during optogenetic stimulation and enabled the detection of strongly coupled neuron pairs at the million-pair and millisecond scales, and thus the inference of patterns of directional information flow. Longitudinal and volumetric measurements of neural couplings may facilitate the study of large-scale neural circuits.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The main data supporting the results of this study are available within the paper and its Supplementary Information. The datasets generated during the study are too large to be publicly shared. Sample data are available from the corresponding authors on reasonable request.
Code availability
Mountainsort3, which we used for spike sorting, is not maintained anymore, but Mountainsort4 is available at https://github.com/magland/ml_ms4alg. Deeplabcut, for animal-posture extraction, is available at https://github.com/DeepLabCut/DeepLabCut. Facemap, for animal-posture extraction, is available at https://github.com/MouseLand/facemap. Visual stimulus decoder was adapted into Matlab code, but Python code is available from the original author at https://github.com/MouseLand/stringer-et-al-2019. BehaveNet for video latent extraction is available at https://github.com/themattinthehatt/behavenet. The LSTM neural decoder is available at https://github.com/KordingLab/Neural_Decoding. The remaining analyses were done with custom Matlab routines, which are available from the corresponding authors on reasonable request.
References
Braitenberg, V. & Schüz, A. Anatomy of the Cortex: Statistics and Geometry (Springer, 1991).
Kleinfeld, D. et al. Can one concurrently record electrical spikes from every neuron in a mammalian brain? Neuron 103, 1005–1015 (2019).
Marblestone, A. H. et al. Physical principles for scalable neural recording. Front. Comput. Neurosci. 7, 137 (2013).
Chen, T. W. et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300 (2013).
Adam, Y. et al. All-optical electrophysiology reveals brain-state dependent changes in hippocampal subthreshold dynamics and excitability. Nature 569, 413 (2019).
Chung, J. E. et al. High-density, long-lasting, and multi-region electrophysiological recordings using polymer electrode arrays. Neuron 101, 21–31.e5 (2019).
Jun, J. J. et al. Fully integrated silicon probes for high-density recording of neural activity. Nature 551, 232 (2017).
Steinmetz, N. A. et al. Neuropixels 2.0: a miniaturized high-density probe for stable, long-term brain recordings. Science 372, eabf4588 (2021).
He, F., Lycke, R., Ganji, M., Xie, C. & Luan, L. Ultraflexible neural electrodes for long-lasting intracortical recording. iScience 23, 101387 (2020).
Luan, L. et al. Ultraflexible nanoelectronic probes form reliable, glial scar-free neural integration. Sci. Adv. 3, e1601966 (2017).
Brown, E. N., Kass, R. E. & Mitra, P. P. Multiple neural spike train data analysis: state-of-the-art and future challenges. Nat. Neurosci. 7, 456–461 (2004).
Wei, X. et al. Nanofabricated ultraflexible electrode arrays for high-density intracortical recording. Adv. Sci. 5, 1700625 (2018).
Zhao, Z. et al. Parallel, minimally-invasive implantation of ultra-flexible neural electrode arrays. J. Neural Eng. 16, 035001 (2019).
Chung, J. E. et al. Chronic implantation of multiple flexible polymer electrode arrays. J. Vis. Exp. 152, e59957 (2019).
Chung, J. E. et al. A fully automated approach to spike sorting. Neuron 95, 1381–1394.e6 (2017).
Siegle, J. H. et al. Survey of spiking in the mouse visual system reveals functional hierarchy. Nature 592, 86–92 (2021).
Niell, C. M. & Stryker, M. P. Highly selective receptive fields in mouse visual cortex. J. Neurosci. 28, 7520–7536 (2008).
Ohki, K., Chung, S., Ch’ng, Y. H., Kara, P. & Reid, R. C. Functional imaging with cellular resolution reveals precise micro-architecture in visual cortex. Nature 433, 597–603 (2005).
Lein, E. S. et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature 445, 168–176 (2007).
Stringer, C., Michaelos, M., Tsyboulski, D., Lindo, S. E. & Pachitariu, M. High-precision coding in visual cortex. Cell 184, 2767–2778.e15 (2021).
Zhao, Z. et al. Nanoelectronic coating enabled versatile multifunctional neural probes. Nano Lett. 17, 4588–4595 (2017).
Nabavi, S. et al. Engineering a memory with LTD and LTP. Nature 511, 348–352 (2014).
Batty, E. et al. BehaveNet: nonlinear embedding and Bayesian neural decoding of behavioral videos. Advances in Neural Information Processing Systems 15706–15717 (2019).
Glaser, J. I. et al. Machine learning for neural decoding. eNeuro 7, ENEURO.0506-19.2020 (2020).
Kozai, T. D. Y. et al. Ultrasmall implantable composite microelectrodes with bioactive surfaces for chronic neural interfaces. Nat. Mater. 11, 1065–1073 (2012).
Berenyi, A. et al. Large-scale, high-density (up to 512 channels) recording of local circuits in behaving animals. J. Neurophysiol. 111, 1132–1149 (2014).
Musk, E. & Neuralink An integrated brain-machine interface platform with thousands of channels. J. Med. Internet Res. 21, e16194 (2019).
Kay, K. et al. Constant sub-second cycling between representations of possible futures in the hippocampus. Cell 180, 552–567.e25 (2020).
Trautmann, E. M. et al. Accurate estimation of neural population dynamics without spike sorting. Neuron 103, 292–308 e294 (2019).
Rolston, J. D., Gross, R. E. & Potter, S. M. Common median referencing for improved action potential detection with multielectrode arrays. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2009, 1604–1607 (2009).
Dhawale, A. K. et al. Automated long-term recording and analysis of neural activity in behaving animals. eLife 6, e27702 (2017).
Combrisson, E. et al. Visbrain: a multi-purpose GPU-accelerated open-source suite for multimodal brain data visualization. Front. Neuroinform. 13, 14 (2019).
Mathis, A. et al. DeepLabCut: markerless pose estimation of user-defined body parts with deep learning. Nat. Neurosci. 21, 1281–1289 (2018).
Stringer, C. et al. Spontaneous behaviors drive multidimensional, brainwide activity. Science 364, 255 (2019).
Acknowledgements
We thank M. Karlsson and M. Borius for input about neural recording electronics, C. Kemere for input about motion decoding, V. Yip for assistance with devices fabrication and assembly and A. Li for assistance with tests of the decoding model. This work was funded by the National Institute of Neurological Disorders and Stroke grants R01NS102917 (to C.X.), U01NS115588 (to C.X. and L.F.), R01NS109361(to L.L.) and UF1 NS107667 (to L.F); the National Heart, Lung and Blood Institute grant K25HL140153 (to L.L.); the Welch Foundation Research grant #F-1941-20170325 (to C.X.); and the Howard Hughes Medical Institute (to L.F.)
Author information
Authors and Affiliations
Contributions
C.X. conceived and organized the overall study; Z.Z., H.Z., X.L., L.L. and C.X. designed the experiments, with inputs from all authors; Z.Z. and X.L. designed and fabricated the NET devices, with supervision from C.X.; D.F.L., J.E.C. and L.F., in collaboration with SpikeGadgets LLC, designed the stacking head-mount recording system; Z.Z. and X.L. designed the NET-probe integration with the head-mount recording system, with help from J.E.C. and D.F.L., and supervision from C.X. and L.F.; Z.Z. and X.L. developed and performed surgical procedures, supervised by C.X.; Z.Z., X.L. and H.Z. performed animal neural-recording experiments, with help from L.S. and F.H., and supervision from C.X. and L.L.; H.Z. and Z.Z. developed and implemented data pre-processing, supervised by C.X. and with input from J.E.C. and L.F.; Z.Z. and H.Z. performed data post-analysis, supervised by L.L. and C.X. and with input from L.F.; Z.Z. performed histology, supervised by C.X.; Z.Z., L.L. and C.X. wrote and revised the manuscript, with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
C.X., L.L. and Z.Z. are co-inventors on a patent filed by The University of Texas (WO2019051163A1, 14 March 2019) on the ultraflexible neural electrode technology described in this study. L. F., L.L. and C.X. hold equity ownership in Neuralthread Inc., an entity that is licensing this technology. All other authors declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
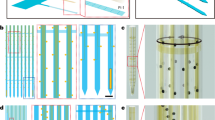
Extended Data Fig. 1 Immunohistochemistry analysis showing the tissue-NET interface at a high implantation density.
One mouse was implanted in visual cortex with 10 type-I NET modules, at inter-shank spacings of 150 µm and inter-module spacings of 250 µm. Fluorescent (a) and brightfield (b) microscopy show no observable scarring in the implanted regions. Neuron: red; NETs: yellow. (c), We counted numbers of neurons in seven randomly-chosen 200-µm-diameter regions centering around NETs (yellow circled regions as an example) and five 200-µm-diameter randomly-chosen regions away from NETs (cyan circled regions as an example). We found no significant difference between the two groups using unpaired T test, p = 0.275 (d), suggesting no significant neuronal loss induced by dense NET implants. These results are consistent with our prior studies using single or few NETs (10, 13). Scalebars: 250 µm (a and b) and 50 µm (c).
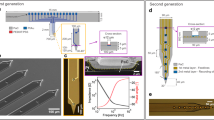
Extended Data Fig. 2 Example recording performances of type-II (A) and –III (B) NET electrode designs.
These two designs offer high electrode density that allows for the recording of individual neurons by multiple channels and therefore perform better in single unit isolation. Color code corresponds to the location of the recording sites. Each dashed box outlines the waveforms of a single unit recorded on the corresponding sites. Scale bars: 100 μV (vertical) and 2 ms (horizontal).
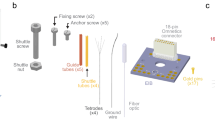
Extended Data Fig. 3 Modular recording system interfacing with NETs.
(a), A ready-to-implant 128-channel NET module connected to a flexible printed circuit (FPC). Left panel shows the NET module and ball grid array (BGA) through which the connection to FPC was made. Right panel shows that individual NET shanks was attached to tungsten microwires for implantation. (b), Photo of a 128-channal stackable headstage connected to NET through an FPC. (c), Photo of a 1024-channel system composed of eight stackable modules. (d), Photo of a free-moving rat carrying a 1024-channel NET array and stackable headstages as shown in c. 3D printed case enclosed all headstages.
Supplementary information
Main Supplementary Information
Supplementary figures, tables and video captions.
Supplementary Video 1
Ultraflexibility of the NET arrays.
Supplementary Video 2
Micro-CT scan of a densely implanted NET array.
Supplementary Video 3
Example of 3D reconstruction of local field potentials recorded by a 1,024-channel NET array.
Supplementary Video 4
Example of spiking activity recorded in visual regions responding to drifting grating stimuli.
Supplementary Video 5
Example of spiking activity recorded by a NET array in visual, sensory and motor cortical regions.
Supplementary Video 6
Example of local field potentials recorded in visual, sensory and motor cortical regions by a NET array.
Supplementary Video 7
Animal facial-motion decoding by large-scale neural recordings.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, Z., Zhu, H., Li, X. et al. Ultraflexible electrode arrays for months-long high-density electrophysiological mapping of thousands of neurons in rodents. Nat. Biomed. Eng 7, 520–532 (2023). https://doi.org/10.1038/s41551-022-00941-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-022-00941-y
This article is cited by
-
T-DOpE probes reveal sensitivity of hippocampal oscillations to cannabinoids in behaving mice
Nature Communications (2024)
-
Soft high-density neural probes enable stable single-neuron recordings
Nature Nanotechnology (2024)
-
Through-polymer, via technology-enabled, flexible, lightweight, and integrated devices for implantable neural probes
Microsystems & Nanoengineering (2024)
-
Translational opportunities and challenges of invasive electrodes for neural interfaces
Nature Biomedical Engineering (2023)
-
Flexible brain–computer interfaces
Nature Electronics (2023)