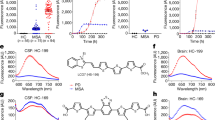
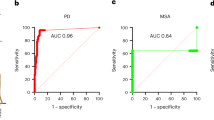
Abstract
Misfolded alpha-synuclein (αSyn) aggregates are a hallmark event in Parkinson’s disease (PD) and other synucleinopathies. Recently, αSyn seed amplification assays (αSyn-SAAs) have shown promise as a test for biochemical diagnosis of synucleinopathies. αSyn-SAAs use the intrinsic self-replicative nature of misfolded αSyn aggregates (seeds) to multiply them in vitro. In these assays, αSyn seeds circulating in biological fluids are amplified by a cyclical process that includes aggregate fragmentation into smaller self-propagating seeds, followed by elongation at the expense of recombinant αSyn (rec-αSyn). Amplification of the seeds allows detection by fluorescent dyes specific for amyloids, such as thioflavin T. Several αSyn-SAA reports have been published in the past under the names ‘protein misfolding cyclic amplification’ (αSyn-PMCA) and ‘real-time quaking-induced conversion’. Here, we describe a protocol for αSyn-SAA, originally reported as αSyn-PMCA, which allows detection of αSyn aggregates in cerebrospinal fluid samples from patients affected by PD, dementia with Lewy bodies or multiple-system atrophy (MSA). Moreover, this αSyn-SAA can differentiate αSyn aggregates from patients with PD versus those from patients with MSA, even in retrospective samples from patients with pure autonomic failure who later developed PD or MSA. We also describe modifications to the original protocol introduced to develop an optimized version of the assay. The optimized version shortens the assay length, decreases the amount of rec-αSyn required and reduces the number of inconclusive results. The protocol has a hands-on time of ~2 h per 96-well plate and can be performed by personnel trained to perform basic experiments with specimens of human origin.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Spillantini, M. G. et al. α-Synuclein in Lewy bodies. Nature 388, 839–840 (1997).
Spillantini, M. G., Crowther, R. A., Jakes, R., Hasegawa, M. & Goedert, M. α-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with Lewy bodies. Proc. Natl Acad. Sci. USA 95, 6469–6473 (1998).
Sulzer, D. & Edwards, R. H. The physiological role of α-synuclein and its relationship to Parkinson’s Disease. J. Neurochem. 150, 475–486 (2019).
Mehra, S., Sahay, S. & Maji, S. K. α-Synuclein misfolding and aggregation: implications in Parkinson’s disease pathogenesis. Biochim. Biophys. Acta Proteins Proteom. 1867, 890–908 (2019).
Steiner, J. A., Quansah, E. & Brundin, P. The concept of alpha-synuclein as a prion-like protein: ten years after. Cell Tissue Res. 373, 161–173 (2018).
Prusiner, S. B. Prions. Proc. Natl Acad. Sci. USA 95, 13363–13383 (1998).
Moda, F. et al. Prions in the urine of patients with variant Creutzfeldt-Jakob disease. N. Engl. J. Med. 371, 530–539 (2014).
Concha-Marambio, L. et al. Detection of prions in blood from patients with variant Creutzfeldt-Jakob disease. Sci. Transl. Med. 8, 370ra183 (2016).
Concha-Marambio, L., Chacon, M. A. & Soto, C. Preclinical detection of prions in blood of nonhuman primates infected with variant Creutzfeldt-Jakob disease. Emerg. Infect. Dis. 26, 34–43 (2020).
Fiorini, M. et al. High diagnostic accuracy of RT-QuIC assay in a prospective study of patients with suspected sCJD. Int. J. Mol. Sci. 21, 880 (2020).
Orrú, C. D. et al. Rapid and sensitive RT-QuIC detection of human Creutzfeldt-Jakob disease using cerebrospinal fluid. mBio 6, e02451-14 (2015).
Orrú, C. D. et al. A test for Creutzfeldt-Jakob disease using nasal brushings. N. Engl. J. Med. 371, 519–529 (2014).
Fairfoul, G. et al. Alpha-synuclein RT-QuIC in the CSF of patients with alpha-synucleinopathies. Ann. Clin. Transl. Neurol. 3, 812–818 (2016).
Shahnawaz, M. et al. Development of a biochemical diagnosis of Parkinson disease by detection of α-synuclein misfolded aggregates in cerebrospinal fluid. JAMA Neurol. 74, 163–172 (2017).
Groveman, B. R. et al. Extended and direct evaluation of RT-QuIC assays for Creutzfeldt-Jakob disease diagnosis. Ann. Clin. Transl. Neurol. 4, 139–144 (2017).
Concha-Marambio, L. et al. Seed amplification assay to diagnose early Parkinson’s and predict dopaminergic deficit progression. Mov. Disord. 36, 2444–2446 (2021).
Russo, M. J. et al. High diagnostic performance of independent alpha-synuclein seed amplification assays for detection of early Parkinson’s disease. Acta Neuropathol. Commun. 9, 179 (2021).
Ferreira, N. D. C. & Caughey, B. Proteopathic seed amplification assays for neurodegenerative disorders. Clin. Lab. Med. 40, 257–270 (2020).
Kang, U. J. et al. Comparative study of cerebrospinal fluid α-synuclein seeding aggregation assays for diagnosis of Parkinson’s disease. Mov. Disord. 34, 536–544 (2019).
Concha-Marambio, L., Shahnawaz, M. & Soto, C. Detection of misfolded α-synuclein aggregates in cerebrospinal fluid by the protein misfolding cyclic amplification platform. Methods Mol. Biol. 1948, 35–44 (2019).
Shahnawaz, M. et al. Discriminating α-synuclein strains in Parkinson’s disease and multiple system atrophy. Nature 578, 273–277 (2020).
Soto, C. & Pritzkow, S. Protein misfolding, aggregation, and conformational strains in neurodegenerative diseases. Nat. Neurosci. 21, 1332–1340 (2018).
Narayanan, A. et al. A first order phase transition mechanism underlies protein aggregation in mammalian cells. Elife 8, 1–26 (2019).
Kocisko, D. A. et al. Cell-free formation of protease-resistant prion protein. Nature 370, 471–474 (1994).
Jarrett, J. T. & Lansbury, P. T. Seeding ‘one-dimensional crystallization’ of amyloid: a pathogenic mechanism in Alzheimer’s disease and scrapie? Cell 73, 1055–1058 (1993).
Saborio, G. P., Permanne, B. & Soto, C. Sensitive detection of pathological prion protein by cyclic amplification of protein misfolding. Nature 411, 810–813 (2001).
Saá, P., Castilla, J. & Soto, C. Ultra-efficient replication of infectious prions by automated protein misfolding cyclic amplification. J. Biol. Chem. 281, 35245–35252 (2006).
Castilla, J., Saá, P. & Soto, C. Detection of prions in blood. Nat. Med. 11, 982–985 (2005).
Saá, P., Castilla, J. & Soto, C. Presymptomatic detection of prions in blood. Science 313, 92–94 (2006).
Bougard, D. et al. Detection of prions in the plasma of presymptomatic and symptomatic patients with variant Creutzfeldt-Jakob disease. Sci. Transl. Med. 8, 370ra182 (2016).
Atarashi, R. et al. Ultrasensitive detection of scrapie prion protein using seeded conversion of recombinant prion protein. Nat. Methods 4, 645–650 (2007).
Atarashi, R. et al. Simplified ultrasensitive prion detection by recombinant PrP conversion with shaking. Nat. Methods 5, 211–212 (2008).
Atarashi, R. et al. Ultrasensitive human prion detection in cerebrospinal fluid by real-time quaking-induced conversion. Nat. Med. 17, 175–178 (2011).
Pujols, J. et al. High-throughput screening methodology to identify alpha-synuclein aggregation inhibitors. Int. J. Mol. Sci. 18, 478 (2017).
Kurnik, M. et al. Potent α-synuclein aggregation inhibitors, identified by high-throughput screening, mainly target the monomeric state. Cell Chem. Biol. 25, 1389–1402.e9 (2018).
Groveman, B. R. et al. Rapid and ultra-sensitive quantitation of disease-associated α-synuclein seeds in brain and cerebrospinal fluid by αSyn RT-QuIC. Acta Neuropathol. Commun. 6, 7 (2018).
Parnetti, L. et al. CSF and blood biomarkers for Parkinson’s disease. Lancet Neurol. 18, 573–586 (2019).
Chen-Plotkin, A. S. et al. Finding useful biomarkers for Parkinson’s disease. Sci. Transl. Med. 10, eaam6003 (2018).
Emamzadeh, F. N. & Surguchov, A. Parkinson’s disease: biomarkers, treatment, and risk factors. Front. Neurosci. 12, 612 (2018).
Sharma, S. et al. Biomarkers in Parkinson’s disease (recent update). Neurochem. Int. 63, 201–229 (2013).
Atik, A., Stewart, T. & Zhang, J. Alpha-synuclein as a biomarker for Parkinson’s disease. Brain Pathol. 26, 410–418 (2016).
Gao, L. et al. Cerebrospinal fluid alpha-synuclein as a biomarker for Parkinson’s disease diagnosis: a systematic review and meta-analysis. Int. J. Neurosci. 125, 645–654 (2015).
Ganguly, U. et al. Alpha-synuclein as a biomarker of Parkinson’s disease: good, but not good enough. Front. Aging Neurosci. 13, 702639 (2021).
Mollenhauer, B. et al. Antibody-based methods for the measurement of α-synuclein concentration in human cerebrospinal fluid—method comparison and round robin study. J. Neurochem. 149, 126–138 (2019).
Ohrfelt, A. et al. Cerebrospinal fluid alpha-synuclein in neurodegenerative disorders—a marker of synapse loss? Neurosci. Lett. 450, 332–335 (2009).
Fujiwara, H. et al. α-Synuclein is phosphorylated in synucleinopathy lesions. Nat. Cell Biol. 4, 160–164 (2002).
Eusebi, P. et al. Diagnostic utility of cerebrospinal fluid α-synuclein in Parkinson’s disease: a systematic review and meta-analysis. Mov. Disord. 32, 1389–1400 (2017).
Conway, K. A. et al. Acceleration of oligomerization, not fibrillization, is a shared property of both alpha-synuclein mutations linked to early-onset Parkinson’s disease: implications for pathogenesis and therapy. Proc. Natl Acad. Sci. USA 97, 571–576 (2000).
Ingelsson, M. Alpha-synuclein oligomers—neurotoxic molecules in Parkinson’s disease and other Lewy body disorders. Front. Neurosci. 10, 408 (2016).
Tokuda, T. et al. Detection of elevated levels of α-synuclein oligomers in CSF from patients with Parkinson disease. Neurology 75, 1766–1772 (2010).
Hansson, O. et al. Levels of cerebrospinal fluid α-synuclein oligomers are increased in Parkinson’s disease with dementia and dementia with Lewy bodies compared to Alzheimer’s disease. Alzheimers Res. Ther. 6, 25 (2014).
Majbour, N. K. et al. Cerebrospinal α-synuclein oligomers reflect disease motor severity in DeNoPa Longitudinal Cohort. Mov. Disord. 36, 2048–2056 (2021).
Han, J.-Y., Jang, H.-S., Green, A. J. E. & Choi, Y. P. RT-QuIC-based detection of alpha-synuclein seeding activity in brains of dementia with Lewy Body patients and of a transgenic mouse model of synucleinopathy. Prion 14, 88–94 (2020).
Manne, S. et al. α-Synuclein real-time quaking-induced conversion in the submandibular glands of Parkinson’s disease patients. Mov. Disord. 35, 268–278 (2020).
Bongianni, M. et al. α-Synuclein RT-QuIC assay in cerebrospinal fluid of patients with dementia with Lewy bodies. Ann. Clin. Transl. Neurol. 6, 2120–2126 (2019).
Han, J.-Y., Shin, C. & Choi, Y. P. Preclinical detection of alpha-synuclein seeding activity in the colon of a transgenic mouse model of synucleinopathy by RT-QuIC. Viruses 13, 759 (2021).
Bargar, C. et al. Streamlined alpha-synuclein RT-QuIC assay for various biospecimens in Parkinson’s disease and dementia with Lewy bodies. Acta Neuropathol. Commun. 9, 62 (2021).
Iranzo, A. et al. Detection of α-synuclein in CSF by RT-QuIC in patients with isolated rapid-eye-movement sleep behaviour disorder: a longitudinal observational study. Lancet Neurol. 20, 203–212 (2021).
De Luca, C. M. G. et al. Efficient RT-QuIC seeding activity for α-synuclein in olfactory mucosa samples of patients with Parkinson’s disease and multiple system atrophy. Transl. Neurodegener. 8, 24 (2019).
Perra, D. et al. Alpha-synuclein seeds in olfactory mucosa and cerebrospinal fluid of patients with dementia with Lewy bodies. Brain Commun. 3, 1–11 (2021).
Stefani, A. et al. Alpha-synuclein seeds in olfactory mucosa of patients with isolated REM sleep behaviour disorder. Brain 144, 1118–1126 (2021).
Singer, W. et al. Alpha-synuclein oligomers and neurofilament light chain in spinal fluid differentiate multiple system atrophy from Lewy Body synucleinopathies. Ann. Neurol. 88, 503–512 (2020).
Wang, Z. et al. Skin α-synuclein aggregation seeding activity as a novel biomarker for Parkinson disease. JAMA Neurol. 78, 1–11 (2020).
Jung, B. C. et al. Amplification of distinct α-synuclein fibril conformers through protein misfolding cyclic amplification. Exp. Mol. Med. 49, e314 (2017).
Fenyi, A. et al. Detection of alpha-synuclein aggregates in gastrointestinal biopsies by protein misfolding cyclic amplification. Neurobiol. Dis. 129, 38–43 (2019).
Rossi, M. et al. Ultrasensitive RT-QuIC assay with high sensitivity and specificity for Lewy body-associated synucleinopathies. Acta Neuropathol. 140, 49–62 (2020).
Park, S.-J. et al. Establishment of method for the determination of aggregated α-synuclein in DLB patient using RT-QuIC assay. Protein Pept. Lett. 28, 115–120 (2021).
Kakuda, K. et al. Ultrasonication-based rapid amplification of α-synuclein aggregates in cerebrospinal fluid. Sci. Rep. 9, 6001 (2019).
Singer, W. et al. Alpha-synuclein oligomers and neurofilament light chain predict phenoconversion of pure autonomic failure. Ann. Neurol. 89, 1212–1220 (2021).
Masuda, M. et al. Cysteine misincorporation in bacterially expressed human α-synuclein. FEBS Lett. 580, 1775–1779 (2006).
Acknowledgements
We thank various members of the laboratory who contributed to the identification and testing of the experimental conditions described here. This work was partially supported by grants from the NIH (R01AG055053, R01AG061069 and R01AG059321) to C.S., R21NS114884 to M.S., and R01 NS119689 to M.S. and S.P., as well as grants from the Michael J. Fox Foundation for Parkinson’s disease to C.S. and S.P., and a grant from the American Parkinson Disease Association to M.S.
Author information
Authors and Affiliations
Contributions
L.C.-M., C.M.F, S.P. and M.S. contributed to the development of the protocols. With the help of S.P., C.M.F. and M.S., L.C.-M. wrote the first draft of the article. C.S. is the principal investigator who provided funding and supervision for this research. C.S. produced the final version of the article.
Corresponding author
Ethics declarations
Competing interests
L.C.-M. and C.M.F. are employees of Amprion Inc., a biotechnology company that focuses on the commercial use of SAAs (PMCA and RT-QuIC) for high-sensitivity detection of misfolded protein aggregates. C.S. is a Founder, Chief Scientific Officer and Member of the Board of Directors of Amprion Inc. The University of Texas Health Science Center at Houston has licensed patents and patent applications to Amprion. The other authors declare no competing interests.
Peer review
Peer review information
Nature Protocols thanks Ehraz Anis, Masato Hasegawa, Saima Zameer and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Shahnawaz, M. et al. Nature 578, 273–277 (2020): https://doi.org/10.1038/s41586-020-1984-7
Shahnawaz, M. et al. JAMA Neurol. 74, 163–172 (2017): https://doi.org/10.1001/jamaneurol.2016.4547
Kang, U. J. et al. Mov. Disord. 34, 536–544 (2019): https://doi.org/10.1002/mds.27646
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Concha-Marambio, L., Pritzkow, S., Shahnawaz, M. et al. Seed amplification assay for the detection of pathologic alpha-synuclein aggregates in cerebrospinal fluid. Nat Protoc 18, 1179–1196 (2023). https://doi.org/10.1038/s41596-022-00787-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-022-00787-3
This article is cited by
-
Cerebrospinal fluid lipoproteins inhibit α-synuclein aggregation by interacting with oligomeric species in seed amplification assays
Molecular Neurodegeneration (2023)
-
Combining skin and olfactory α-synuclein seed amplification assays (SAA)—towards biomarker-driven phenotyping in synucleinopathies
npj Parkinson's Disease (2023)
-
A seed amplification assay to detect Parkinson disease pathology
Nature Reviews Neurology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.