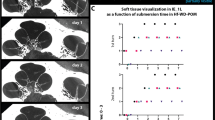
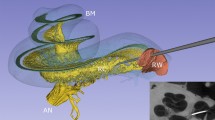
Abstract
In vivo and real-time multicellular imaging enables the decoding of sensory circuits and the tracking of systemic drug uptake. However, in vivo imaging of the auditory periphery remains technically challenging owing to the deep location, mechanosensitivity and fluid-filled, bone-encased nature of the cochlear structure. Existing methods that expose the cochlea invariably cause irreversible damage to auditory function, severely limiting the experimental measurements possible in living animals. Here we present an in vivo surgical protocol that permits the imaging of cochlear cells in hearing mice. Our protocol describes a ventro-lateral approach for preserving external and middle ear structures while performing surgery, the correct mouse positioning for imaging cochlear cells with effective sound transmission into the ear, the chemo-mechanical cochleostomy for creating the imaging window in the otic capsule bone that prevents intracochlear fluid leakage by maintaining an intact endosteum, and the release of intracochlear pressure that separates the endosteum from the otic capsule bone while creating an imaging window. The procedure thus preserves hearing thresholds. Individual inner and outer hair cells, supporting cells and nerve fibers can be visualized in vivo while hearing function is preserved. This approach may enable future original investigations, such as the real-time tracking of ototoxic drug transport into the cochleae. The technique may be applied to the monitoring of sound-evoked functional activity in multiple cochlear cells, in combination with optogenetic tools, and may help to improve cochlear implantation in humans. The cochleostomy takes ~1 h and requires experience in surgery.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
Data availability
The authors declare that the main data discussed in this protocol are available in the supporting primary research paper (https://www.pnas.org/doi/10.1073/pnas.2117946119).
References
Yin, L. et al. Imaging light responses of retinal ganglion cells in the living mouse eye. J. Neurophysiol. 109, 2415–2421 (2013).
Charpak, S., Mertz, J., Beaurepaire, E., Moreaux, L. & Delaney, K. Odor-evoked calcium signals in dendrites of rat mitral cells. Proc. Natl Acad. Sci. USA 98, 1230–1234 (2001).
Barretto, R. P. J. et al. The neural representation of taste quality at the periphery. Nature 517, 373–U511 (2015).
Kim, Y. S. et al. Coupled activation of primary sensory neurons contributes to chronic pain. Neuron 91, 1085–1096 (2016).
Imamura, S. & Adams, J. C. Distribution of gentamicin in the guinea pig inner ear after local or systemic application. J. Assoc. Res. Otolaryngol. 4, 176–195 (2003).
Wang, Q. & Steyger, P. S. Trafficking of systemic fluorescent gentamicin into the cochlea and hair cells. J. Assoc. Res. Otolaryngol. 10, 205–219 (2009).
Tanoshima, R. et al. Analyses of adverse drug reactions-nationwide active surveillance network: Canadian Pharmacogenomics Network for Drug Safety Database. J. Clin. Pharmacol. 59, 356–363 (2019).
Du, X. et al. A hand-guided robotic drill for cochleostomy on human cadavers. Robot Surg. 5, 13–18 (2018).
Fishman, A. J., Moreno, L. E., Rivera, A. & Richter, C. P. CO2 laser fiber soft cochleostomy: development of a technique using human temporal bones and a guinea pig model. Laser Surg. Med. 42, 245–256 (2010).
Ren, D. D. & Chi, F. L. Experimental study on thermic effects, morphology and function of guinea pig cochlea: a comparison between the erbium: yttrium–aluminum–garnet laser and carbon dioxide laser. Laser Surg. Med. 40, 407–414 (2008).
Cipolla, M. J., Iyer, P., Dome, C., Welling, D. B. & Bush, M. L. Modification and comparison of minimally invasive cochleostomy techniques: a pilot study. Laryngoscope 122, 1142–1147 (2012).
Kiefer, J. et al. Conservation of low-frequency hearing in cochlear implantation. Acta Oto-Laryngol. 124, 272–280 (2004).
Dong, W. & Cooper, N. P. An experimental study into the acousto-mechanical effects of invading the cochlea. J. R. Soc. Interface 3, 561–571 (2006).
Cooper, N. P. & Rhode, W. S. Fast travelling waves, slow travelling waves and their interactions in experimental studies of apical cochlear mechanics. Audit Neurosci. 2, 289–299 (1996).
Ulfendahl, M., Khanna, S. M. & Flock, A. Effects of opening and resealing the cochlea on the mechanical response in the isolated temporal bone preparation. Hearing Res. 57, 31–37 (1991).
Alyono, J. C., Corrales, C. E., Huth, M. E., Blevins, N. H. & Ricci, A. J. Development and characterization of chemical cochleostomy in the guinea pig. Otolaryngol. Head. Neck Surg. 152, 1113–1118 (2015).
Kim, J. & Ricci, A.J. In vivo real-time imaging reveals megalin as the aminoglycoside gentamicin transporter into cochlea whose inhibition is otoprotective. Proc. Natl Acad. Sci. USA 119, e2117946119 (2022).
Lee, H. Y. et al. Noninvasive in vivo imaging reveals differences between tectorial membrane and basilar membrane traveling waves in the mouse cochlea. Proc. Natl Acad. Sci. USA 112, 3128–3133 (2015).
Nuttall, A. L., Dolan, D. F. & Avinash, G. Laser Doppler velocimetry of basilar membrane vibration. Hear. Res. 51, 203–213 (1991).
Ruggero, M. A. & Rich, N. C. Application of a commercially-manufactured Doppler-shift laser velocimeter to the measurement of basilar-membrane vibration. Hear. Res. 51, 215–230 (1991).
Ren, T. Longitudinal pattern of basilar membrane vibration in the sensitive cochlea. Proc. Natl Acad. Sci. USA 99, 17101–17106 (2002).
Ren, T. Reverse propagation of sound in the gerbil cochlea. Nat. Neurosci. 7, 333–334 (2004).
Dong, W. & Olson, E. S. Detection of cochlear amplification and its activation. Biophys. J. 105, 1067–1078 (2013).
Dallos, P. Response characteristics of mammalian cochlear hair cells. J. Neurosci. 5, 1591–1608 (1985).
Russell, I. J. & Sellick, P. M. Low-frequency characteristics of intracellularly recorded receptor potentials in guinea-pig cochlear hair cells. J. Physiol. 338, 179–206 (1983).
Liberman, M. C. Auditory-nerve response from cats raised in a low-noise chamber. J. Acoust. Soc. Am. 63, 442–455 (1978).
Lee, H. Y. et al. Two-dimensional cochlear micromechanics measured in vivo demonstrate radial tuning within the mouse organ of Corti. J. Neurosci. 36, 8160–8173 (2016).
Wang, R. K. & Nuttall, A. L. Phase-sensitive optical coherence tomography imaging of the tissue motion within the organ of Corti at a subnanometer scale: a preliminary study. J. Biomed. Opt. 15, 056005 (2010).
Jawadi, Z., Applegate, B. E. & Oghalai, J. S. Optical coherence tomography to measure sound-induced motions within the mouse organ of Corti in vivo. Methods Mol. Biol. 1427, 449–462 (2016).
Kim, J., Xia, A., Grillet, N., Applegate, B. E. & Oghalai, J. S. Osmotic stabilization prevents cochlear synaptopathy after blast trauma. Proc. Natl Acad. Sci. USA 115, E4853–E4860 (2018).
McGinley, M. J., Liberman, M. C., Bal, R. & Oertel, D. Generating synchrony from the asynchronous: compensation for cochlear traveling wave delays by the dendrites of individual brainstem neurons. J. Neurosci. 32, 9301–9311 (2012).
Marker, D.F., Tremblay, M.E., Lu, S.M., Majewska, A.K. & Gelbard, H.A. A thin-skull window technique for chronic two-photon in vivo imaging of murine microglia in models of neuroinflammation. J. Vis. Exp. 43, 2059 (2010).
Mancini, M. et al. Head and neck veins of the mouse. a magnetic resonance, micro computed tomography and high frequency color Doppler ultrasound study. PLoS ONE 10, e0129912 (2015).
Acknowledgements
Our thanks to those involved in the early stages of development including N. Blevins, E. Corrales and J. C. Alyono. We are also grateful to J. B. Azimzadeh, who commented on the manuscript, C. Gralapp, who drew artworks, and E. Scheibinger, who helped to list materials. Lastly, we thank J. S. Oghalai for having shared a mouse head holder and the surgical approach to the cochlea through the bulla. This project was funded by National Institutes of Health grants R01 DC014720 and DC003896-16. Our thanks for philanthropic contributions from the Stanford Initiative to Cure Hearing Loss and the generous donations of the Oberndorf Foundation.
Author information
Authors and Affiliations
Contributions
J.K. and A.J.R. designed the experiments and developed the mouse cochleostomy; J.K. performed mouse surgery, ABR test, in vivo two-photon imaging and data analysis; J.K. and A.J.R. wrote the manuscript; A.J.R. supervised the entire project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Protocols thanks David Corey and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key reference using this protocol
Kim, J. & Ricci, A. Proc. Natl Acad. Sci. USA 119, e2117946119 (2022): https://doi.org/10.1073/pnas.2117946119
Supplementary information
Supplementary Video 1
Imaging window creation
Supplementary Data 1
Mouse head holder, part1.
Supplementary Data 2
Mouse head holder, part2.
Supplementary Data 3
Mouse head holder, part3.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, J., Ricci, A.J. A chemo-mechanical cochleostomy preserves hearing for the in vivo functional imaging of cochlear cells. Nat Protoc 18, 1137–1154 (2023). https://doi.org/10.1038/s41596-022-00786-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-022-00786-4
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.