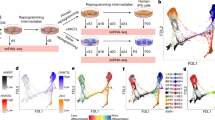
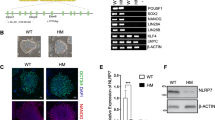
Abstract
Cell reprogramming has allowed unprecedented access to human development, from virtually any genome. However, reprogramming yields pluripotent stem cells that can differentiate into all cells that form a fetus, but not extraembryonic annexes. Therefore, a cellular model allowing study of placental development from a broad genomic repertoire is lacking. Here, we describe an optimized protocol to reprogram somatic cells into human induced trophoblast stem cells (hiTSCs) and convert pluripotent stem cells into human converted TSCs (hcTSCs). This protocol enables much-needed genome-specific placental disease modeling. We also detail extravillous trophoblast and syncytiotrophoblast differentiation protocols from hiTSCs and hcTSCs, a necessary step to validate these cells. In total, this protocol takes 4 months and requires advanced cell culture skills, comparable to those necessary for somatic cell reprogramming into human induced pluripotent stem cells.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All datasets used in this paper were uploaded to the European Nucleotide Archive (https://www.ebi.ac.uk/ena/browser/view/PRJEB34037?show=reads), as specified in our previous paper5.
References
Okae, H. et al. Derivation of human trophoblast stem cells. Cell Stem Cell 22, 50–63.e6 (2018).
Haider, S. et al. Self-renewing trophoblast organoids recapitulate the developmental program of the early human placenta. Stem Cell Rep. 11, 537–551 (2018).
Turco, M. Y. et al. Trophoblast organoids as a model for maternal-fetal interactions during human placentation. Nature 564, 263–267 (2018).
Saha, B. et al. TEAD4 ensures postimplantation development by promoting trophoblast self-renewal: an implication in early human pregnancy loss. Proc. Natl Acad. Sci. USA 117, 17864–17875 (2020).
Castel, G. et al. Induction of human trophoblast stem cells from somatic cells and pluripotent stem cells. Cell Rep. 33, 108419 (2020).
Amita, M. et al. Complete and unidirectional conversion of human embryonic stem cells to trophoblast by BMP4. Proc. Natl Acad. Sci. USA 110, E1212–E1221 (2013).
Krendl, C. et al. GATA2/3-TFAP2A/C transcription factor network couples human pluripotent stem cell differentiation to trophectoderm with repression of pluripotency. Proc. Natl Acad. Sci. USA 114, E9579–E9588 (2017).
Horii, M. et al. Human pluripotent stem cells as a model of trophoblast differentiation in both normal development and disease. Proc. Natl Acad. Sci. USA 113, E3882–E3891 (2016).
Horii, M. et al. Modeling preeclampsia using human induced pluripotent stem cells. Sci. Rep. 11, 5877 (2021).
Sheridan, M. A. et al. Early onset preeclampsia in a model for human placental trophoblast. Proc. Natl Acad. Sci. USA 116, 4336–4345 (2019).
Takashima, Y. et al. Resetting transcription factor control circuitry toward ground-state pluripotency in human. Cell 158, 1254–1269 (2014).
Yang, Y. et al. Derivation of pluripotent stem cells with in vivo embryonic and extraembryonic potency. Cell 169, 243–257.e25 (2017).
Amit, M. et al. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev. Biol. 227, 271–278 (2000).
Rossant, J. & Tam, P. P. L. Opportunities and challenges with stem cell-based embryo models. Stem Cell Rep. 16, 1031–1038 (2021).
Zhou, J. et al. Modeling human peri-implantation placental development and function. Biol. Reprod. 105, 40–51 (2021).
Horii, M., Touma, O., Bui, T. & Parast, M. M. Modeling human trophoblast, the placental epithelium at the maternal fetal interface. Reproduction 160, R1–R11 (2020).
Moser, G., Weiss, G., Gauster, M., Sundl, M. & Huppertz, B. Evidence from the very beginning: endoglandular trophoblasts penetrate and replace uterine glands in situ and in vitro. Hum. Reprod. 30, 2747–2757 (2015).
Moser, G. et al. Extravillous trophoblasts invade more than uterine arteries: evidence for the invasion of uterine veins. Histochem. Cell Biol. 147, 353–366 (2017).
Moser, G., Drewlo, S., Huppertz, B. & Armant, D. R. Trophoblast retrieval and isolation from the cervix: origins of cervical trophoblasts and their potential value for risk assessment of ongoing pregnancies. Hum. Reprod. Update 24, 484–496 (2018).
Papuchova, H. et al. Three types of HLA-G+ extravillous trophoblasts that have distinct immune regulatory properties. Proc. Natl Acad. Sci. USA 117, 15772–15777 (2020).
Pollheimer, J., Vondra, S., Baltayeva, J., Beristain, A. G. & Knöfler, M. Regulation of placental extravillous trophoblasts by the maternal uterine environment. Front. Immunol. 9, 2597 (2018).
Borbely, A. U. et al. The term basal plate of the human placenta as a source of functional extravillous trophoblast cells. Reprod. Biol. Endocrinol. 12, 7 (2014).
Dong, C. et al. Derivation of trophoblast stem cells from naïve human pluripotent stem cells. Elife 9, e52504 (2020).
Genbacev, O., Joslin, R., Damsky, C. H., Polliotti, B. M. & Fisher, S. J. Hypoxia alters early gestation human cytotrophoblast differentiation/invasion in vitro and models the placental defects that occur in preeclampsia. J. Clin. Invest. 97, 540–550 (1996).
Liu, X. et al. Reprogramming roadmap reveals route to human induced trophoblast stem cells. Nature 586, 101–107 (2020).
Tan, J. P., Liu, X. & Polo, J. M. Establishment of human induced trophoblast stem cells via reprogramming of fibroblasts Nat. Protoc. in press.
Cinkornpumin, J. K. et al. Naive human embryonic stem cells can give rise to cells with a trophoblast-like transcriptome and methylome. Stem Cell Rep. 15, 198–213 (2020).
Guo, G. et al. Human naive epiblast cells possess unrestricted lineage potential. Cell Stem Cell 28, 1040–1056.e6 (2021).
Gao, X. et al. Establishment of porcine and human expanded potential stem cells. Nat. Cell Biol. 21, 687–699 (2019).
Kagawa, H. et al. Human blastoids model blastocyst development and implantation. Nature 601, 600–605 (2022).
Liu, X. et al. Modelling human blastocysts by reprogramming fibroblasts into iBlastoids. Nature 591, 627–632 (2021).
Sozen, B. et al. Reconstructing aspects of human embryogenesis with pluripotent stem cells. Nat. Commun. 12, 5550 (2021).
Yanagida, A. et al. Naive stem cell blastocyst model captures human embryo lineage segregation. Cell Stem Cell 28, 1016–1022.e4 (2021).
Yu, L. et al. Blastocyst-like structures generated from human pluripotent stem cells. Nature 591, 620–626 (2021).
Kudo, Y. et al. Quantifying the syncytialisation of human placental trophoblast BeWo cells grown in vitro. Biochim. Biophys. Acta 1640, 25–31 (2003).
Wice, B., Menton, D., Geuze, H. & Schwartz, A. L. Modulators of cyclic AMP metabolism induce syncytiotrophoblast formation in vitro. Exp. Cell Res. 186, 306–316 (1990).
David, L. & Polo, J. M. Phases of reprogramming. Stem Cell Res. 12, 754–761 (2014).
Takahashi, S. et al. Loss of p57KIP2 expression confers resistance to contact inhibition in human androgenetic trophoblast stem cells. Proc. Natl Acad. Sci. USA 116, 26606–26613 (2019).
Jozefczuk, J., Drews, K. & Adjaye, J. Preparation of mouse embryonic fibroblast cells suitable for culturing human embryonic and induced pluripotent stem cells. J. Vis. Exp. 2012, 3854 (2012).
Kilens, S. et al. Parallel derivation of isogenic human primed and naive induced pluripotent stem cells. Nat. Commun. 9, 360 (2018).
Acknowledgements
G.C. is a recipient of an ‘INSERM-Région Pays de la Loire’ fellowship. We acknowledge the MicroPICell, GenoA, BIRD and iPSC core facilities, all supported by Biogenouest and IBiSA, for the use of their resources and technical support. MicroPICell is a member of the national infrastructure France-Bioimaging (ANR-10-INBS-04). BIRD is a member of Institut Français de Bioinformatique (IFB) (ANR-11-INBS-0013). We thank M. Narimatsu for the tips regarding coverslip preparation.
Author information
Authors and Affiliations
Contributions
G.C. and L.D. designed experiments, and G.C. conducted them. G.C. and L.D. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Protocols thanks Berthold Huppertz and Michael Roberts for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key reference using this protocol
Castel, G. et al. Cell Rep. 33, 108419 (2020): https://doi.org/10.1016/j.celrep.2020.108419
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Castel, G., David, L. Induction of human trophoblast stem cells. Nat Protoc 17, 2760–2783 (2022). https://doi.org/10.1038/s41596-022-00744-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-022-00744-0
This article is cited by
-
Establishment of human induced trophoblast stem cells via reprogramming of fibroblasts
Nature Protocols (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.