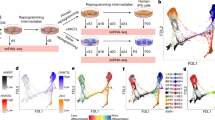
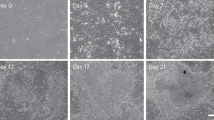
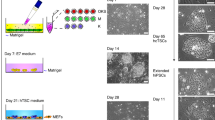
Abstract
During early mammalian embryonic development, trophoblast cells play an essential role in establishing cell–cell interactions at the maternal–fetal interface to ensure a successful pregnancy. In a recent study, we showed that human fibroblasts can be reprogrammed into induced trophoblast stem (iTS) cells by transcription factor-mediated nuclear reprogramming using the Yamanaka factors OCT4, KLF4, SOX2 and c-MYC (OKSM) and a selection of TS cell culture conditions. The derivation of TS cells from human blastocysts or first-trimester placenta can be limited by difficulties in obtaining adequate material as well as ethical implications. By contrast, the described approach allows the generation of iTS cells from the adult cells of individuals with diverse genetic backgrounds, which are readily accessible to many laboratories around the world. Here we describe a step-by-step protocol for the generation and establishment of human iTS cells directly from dermal fibroblasts using a non-integrative reprogramming method. The protocol consists of four main sections: (1) recovery of cryopreserved human dermal fibroblasts, (2) somatic cell reprogramming, (3) passaging of reprogramming intermediates and (4) derivation of iTS cell cultures followed by routine maintenance of iTS cells. These iTS cell lines can be established in 2–3 weeks and cultured long term over 50 passages. We also discuss several characterization methods that can be performed to validate the iTS cells derived using this approach. Our protocol allows researchers to generate patient-specific iTS cells to interrogate the trophoblast and placenta biology as well as their interactions with embryonic cells in health and diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The main data discussed in this protocol were generated as part of the studies published in the supporting primary research paper15.
References
Thomson, J. A. et al. Embryonic stem cell lines derived from human blastocysts. Science 282, 1145–1147 (1998).
Takahashi, K. et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 (2007).
Yu, J. et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 318, 1917–1920 (2007).
Orendi, K. et al. Placental and trophoblastic in vitro models to study preventive and therapeutic agents for preeclampsia. Placenta 32 (Suppl.), S49–S54 (2011).
Sooranna, S. R., Oteng-Ntim, E., Meah, R., Ryder, T. A. & Bajoria, R. Characterization of human placental explants: morphological, biochemical and physiological studies using first and third trimester placenta. Hum. Reprod. 14, 536–541 (1999).
Turco, M. Y. & Moffett, A. Development of the human placenta. Development 146, dev163428 (2019).
Heazlewood, C. F. et al. High incidence of contaminating maternal cell overgrowth in human placental mesenchymal stem/stromal cell cultures: a systematic review. Stem Cells Transl. Med. 3, 1305–1311 (2014).
Apps, R. et al. Genome-wide expression profile of first trimester villous and extravillous human trophoblast cells. Placenta 32, 33–43 (2011).
Pattillo, R. A. & Gey, G. O. The establishment of a cell line of human hormone-synthesizing trophoblastic cells in vitro. Cancer Res. 28, 1231–1236 (1968).
Kohler, P. O. & Bridson, W. E. Isolation of hormone-producing clonal lines of human choriocarcinoma. J. Clin. Endocrinol. Metab. 32, 683–687 (1971).
Hertz, R. Choriocarcinoma of women maintained in serial passage in hamster and rat. Proc. Soc. Exp. Biol. Med. 102, 77–81 (1959).
Amita, M. et al. Complete and unidirectional conversion of human embryonic stem cells to trophoblast by BMP4. Proc. Natl Acad. Sci. Us. A 110, E1212–E1221 (2013).
Xu, R.-H. et al. BMP4 initiates human embryonic stem cell differentiation to trophoblast. Nat. Biotechnol. 20, 1261–1264 (2002).
Okae, H. et al. Derivation of human trophoblast stem cells. Cell Stem Cell 22, 50–63.e6 (2018).
Liu, X. et al. Reprogramming roadmap reveals route to human induced trophoblast stem cells. Nature 586, 101–107 (2020).
Mischler, A., Karakis, V., Mahinthakumar, J. & Carberry, C. Two distinct trophectoderm lineage stem cells from human pluripotent stem cells. J. Biol. Chem. 296, 100386 (2021).
Wei, Y. et al. Efficient derivation of human trophoblast stem cells from primed pluripotent stem cells. Sci Adv 7, (2021).
Li, Z., Kurosawa, O. & Iwata, H. Establishment of human trophoblast stem cells from human induced pluripotent stem cell-derived cystic cells under micromesh culture. Stem Cell Res. Ther. 10, 245 (2019).
Dong, C. et al. Derivation of trophoblast stem cells from naïve human pluripotent stem cells. eLife 9, e52504 (2020).
Cinkornpumin, J. K. et al. Naive human embryonic stem cells can give rise to cells with a trophoblast-like transcriptome and methylome. Stem Cell Rep. 15, 198–213 (2020).
Guo, G. et al. Human naive epiblast cells possess unrestricted lineage potential. Cell. Stem Cell. 28, 1040–1056.e6 (2021).
Io, S. et al. Capturing human trophoblast development with naive pluripotent stem cells in vitro. Cell Stem Cell 28, 1023–1039.e13 (2021).
Castel, G. et al. Induction of human trophoblast stem cells from somatic cells and pluripotent stem cells. Cell Rep. 33, 108419 (2020).
Bayerl, J. et al. Principles of signaling pathway modulation for enhancing human naive pluripotency induction. Cell Stem Cell 28, 1549–1565.e12 (2021).
Castel, G. & David, L. Induction of human trophoblast stem cells. Nat. Protoc. https://doi.org/10.1038/s41596-022-00744-0 (2022).
Chang, C.-W., Wakeland, A. K. & Parast, M. M. Trophoblast lineage specification, differentiation and their regulation by oxygen tension. J. Endocrinol. 236, R43–R56 (2018).
Scifres, C. M. & Nelson, D. M. Intrauterine growth restriction, human placental development and trophoblast cell death. J. Physiol. 587, 3453–3458 (2009).
Sheridan, M. A. et al. Early onset preeclampsia in a model for human placental trophoblast. Proc. Natl Acad. Sci. USA 116, 4336–4345 (2019).
Alici-Garipcan, A., Özçimen, B., Suder, I. & Ülker, V. NLRP7 plays a functional role in regulating BMP4 signaling during differentiation of patient-derived trophoblasts. bioRxiv (2019).
Horii, M. et al. Human pluripotent stem cells as a model of trophoblast differentiation in both normal development and disease. Proc. Natl Acad. Sci. USA 113, E3882–E3891 (2016).
Takahashi, S. et al. Loss of p57KIP2 expression confers resistance to contact inhibition in human androgenetic trophoblast stem cells. Proc. Natl Acad. Sci. USA https://doi.org/10.1073/pnas.1916019116 (2019).
Yu, J. et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science 324, 797–801 (2009).
Warren, L. et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell 7, 618–630 (2010).
Turco, M. Y. et al. Trophoblast organoids as a model for maternal-fetal interactions during human placentation. Nature 564, 263–267 (2018).
Haider, S. et al. Self-renewing trophoblast organoids recapitulate the developmental program of the early human placenta. Stem Cell Rep. 11, 537–551 (2018).
Paynter, J. M., Chen, J., Liu, X. & Nefzger, C. M. Propagation and maintenance of mouse embryonic stem cells. Methods Mol. Biol. 1940, 33–45 (2019).
Liu, X. et al. Generation of mouse-induced pluripotent stem cells by lentiviral transduction. Methods Mol. Biol. 1940, 63–76 (2019).
CytoTuneTM-iPS 2.0 Sendai Reprogramming Kit. https://www.thermofisher.com/order/catalog/product/A16517 (2022).
Muir, A., Lever, A. M. L. & Moffett, A. Human endogenous retrovirus-W envelope (syncytin) is expressed in both villous and extravillous trophoblast populations. J. Gen. Virol. 87, 2067–2071 (2006).
Lee, C. Q. E. et al. What is trophoblast? A combination of criteria define human first-trimester trophoblast. Stem Cell Rep. 6, 257–272 (2016).
Acknowledgements
The authors thank the staff at Monash Flowcore Facility for providing high-quality cell sorting service and technical input. The authors acknowledge the use of the services and facilities of Micromon, Monash Micro Imaging and Monash Histology Platforms at Monash University. We also thank J. Hatwell-Humble and S. Nilsson for assistance with the mouse work. The schematics were created with BioRender.com. This work was supported by National Health and Medical Research Council (NHMRC) project grants APP1104560 and APP2004774 to J.M.P.; a Silvia and Charles Viertel Senior Medical Research Fellowship; and an ARC Future Fellowship FT180100674. J.P.T. was supported by a Monash International Tuition Scholarship and Research Training Program Scholarship. X.L. was supported by the Monash International Postgraduate Research Scholarship, a Monash Graduate Scholarship and the Carmela and Carmelo Ridolfo Prize in Stem Cell Research. The Australian Regenerative Medicine Institute is supported by grants from the State Government of Victoria and the Australian Government.
Author information
Authors and Affiliations
Contributions
J.P.T. and X.L. drafted the protocol. J.P.T., X.L. and J.M.P. wrote the protocol. Most experiments presented in this protocol were performed by J.P.T. with supervision from X.L. and J.M.P.
Corresponding authors
Ethics declarations
Competing interests
Although not directly related to this paper, J.M.P. is a co-founder and shareholder of Mogrify Ltd., a cell therapy company. X.L. and J.M.P. are co-inventors on a PCT patent application (application number 2019904283) filed by Monash University, National University of Singapore and Université de Nantes related to work on derivation of iTS cells. The other authors declare no competing interests.
Peer review
Peer review information
Nature Protocols thanks Robert Morey, Kaela Varberg and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key reference using this protocol
Liu, X et al. Nature 586, 101–107 (2020): https://doi.org/10.1038/s41586-020-2734-6
Supplementary information
Supplementary Information
Flow cytometry gating strategy for live cells
Source data
Source Data Fig. 4
Values of the relative expression for each gene in Fig. 4b.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tan, J.P., Liu, X. & Polo, J.M. Establishment of human induced trophoblast stem cells via reprogramming of fibroblasts. Nat Protoc 17, 2739–2759 (2022). https://doi.org/10.1038/s41596-022-00742-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-022-00742-2
This article is cited by
-
Reprogramming fibroblast into human iBlastoids
Nature Protocols (2024)
-
A comprehensive review of human trophoblast fusion models: recent developments and challenges
Cell Death Discovery (2023)
-
A placental model of SARS-CoV-2 infection reveals ACE2-dependent susceptibility and differentiation impairment in syncytiotrophoblasts
Nature Cell Biology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.