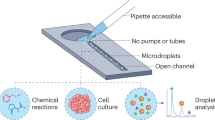
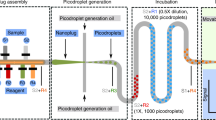
Abstract
Droplet microfluidics is a powerful tool for a variety of biological applications including single-cell genetics, antibody discovery and directed evolution. All these applications make use of genetic libraries, illustrating the difficulty of generating chemically distinct droplets for screening applications. This protocol describes our Braille Display valving platform for on-demand generation of droplets with different chemical contents (16 different reagents and combinations thereof), as well as sorting droplets with different chemical properties, on the basis of fluorescence signals. The Braille Display platform is compact, versatile and cost efficient (only ~US$1,000 on top of a standard droplet microfluidics setup). The procedure includes manufacturing of microfluidic chips, assembly of custom hardware, co-encapsulation of cells and drugs into droplets, fluorescence detection of readout signals and data analysis using shared, freely available LabVIEW and Python packages. As a first application, we demonstrate the complete workflow for screening cancer cell drug sensitivities toward 74 conditions. Furthermore, we describe here an assay enabling the normalization of the observed drug sensitivity to the number of cancer cells per droplet, which additionally increases the robustness of the system. As a second application, we also demonstrate the sorting of droplets according to enzymatic activity. The drug screening application can be completed within 2 d; droplet sorting takes ~1 d; and all preparatory steps for manufacturing molds, chips and setting up the Braille controller can be accomplished within 1 week.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Data are available within the paper; extended data or information can be found on GitHub (https://github.com/saezlab/plugy) or on Zenodo (https://doi.org/10.5281/zenodo.1248886). Specific information can also be provided upon request. All software, CAD design files and analysis tools for running the BD platform are provided with this paper. Source data are provided with this paper.
Code availability
The software repository can be found at https://github.com/saezlab/plugy. For getting the terminal please refer to https://git-scm.com/download/win, and for troubleshooting, we recommend the guide at https://github.com/saezlab/plugy/notebooks/plugy_guide.html.
References
Eduati, F. et al. A microfluidics platform for combinatorial drug screening on cancer biopsies. Nat. Commun. 9, 2434 (2018).
Zec, H., Rane, T. D. & Wang, T. H. Microfluidic platform for on-demand generation of spatially indexed combinatorial droplets. Lab Chip 12, 3055–3062 (2012).
Miller, O. J. et al. High-resolution dose–response screening using droplet-based microfluidics. Proc. Natl Acad. Sci. USA 109, 378–383 (2012).
Dressler, O. J., Maceiczyk, R. M., Chang, S. I. & deMello, A. J. Droplet-based microfluidics: enabling impact on drug discovery. J. Biomol. Screen. 19, 483–496 (2014).
Clausell-Tormos, J., Griffiths, A. D. & Merten, C. A. An automated two-phase microfluidic system for kinetic analyses and the screening of compound libraries. Lab Chip 10, 1302–1307 (2010).
Macosko, E. Z. et al. Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell 161, 1202–1214 (2015).
Klein, A. M. et al. Droplet barcoding for single-cell transcriptomics applied to embryonic stem cells. Cell 161, 1187–1201 (2015).
Stoeckius, M. et al. Cell hashing with barcoded antibodies enables multiplexing and doublet detection for single cell genomics. Genome Biol. 19, 224–224 (2018).
Lyu, F., Blauch, L. R. & Tang, S. K. Y. Quantifying phenotypes in single cells using droplet microfluidics. Methods Cell Biol. 148, 133–159 (2018).
El Debs, B., Utharala, R., Balyasnikova, I. V., Griffiths, A. D. & Merten, C. A. Functional single-cell hybridoma screening using droplet-based microfluidics. Proc. Natl Acad. Sci. USA 109, 11570–11575 (2012).
Shembekar, N., Hu, H., Eustace, D. & Merten, C. A. Single-cell droplet microfluidic screening for antibodies specifically binding to target cells. Cell Rep. 22, 2206–2215 (2018).
Gielen, F. et al. A fully unsupervised compartment-on-demand platform for precise nanoliter assays of time-dependent steady-state enzyme kinetics and inhibition. Anal. Chem. 85, 4761–4769 (2013).
Kulesa, A., Kehe, J., Hurtado, J. E., Tawde, P. & Blainey, P. C. Combinatorial drug discovery in nanoliter droplets. Proc. Natl Acad. Sci. USA 115, 6685–6690 (2018).
Ferraro, D. et al. Droplet microfluidic and magnetic particles platform for cancer typing. Methods Mol. Biol. 1547, 113–121 (2017).
Gu, W., Zhu, X., Futai, N., Cho, B. S. & Takayama, S. Computerized microfluidic cell culture using elastomeric channels and Braille displays. Proc. Natl Acad. Sci. USA 101, 15861–15866 (2004).
Gruner, P. et al. Controlling molecular transport in minimal emulsions. Nat. Commun. 7, 10392 (2016).
Clausell-Tormos, J. et al. Droplet-based microfluidic platforms for the encapsulation and screening of mammalian cells and multicellular organisms. Chem. Biol. 15, 875–875 (2008).
Utharala, R., Tseng, Q., Furlong, E. E. M. & Merten, C. A. A versatile, low-cost, multiway microfluidic sorter for droplets, cells, and embryos. Anal. Chem. 90, 5982–5988 (2018).
Eduati, F. et al. Patient-specific logic models of signaling pathways from screenings on cancer biopsies to prioritize personalized combination therapies. Mol. Syst. Biol. 16, e8664 (2020).
Mathur, L., Ballinger, M., Utharala, R. & Merten, C. A. Microfluidics as an enabling technology for personalized cancer therapy. Small 16, e1904321 (2020).
Dietrich, S. et al. Drug-perturbation-based stratification of blood cancer. J. Clin. Invest. 128, 427–445 (2018).
Mathur, L. et al. Combi-Seq: multiplexed transcriptome-based profiling of drug combinations using deterministic barcoding in single-cell droplets. Nat. Commun. 13, 4450 (2022).
Wu, A. R., Wang, J., Streets, A. M. & Huang, Y. Single-cell transcriptional analysis. Annu. Rev. Anal. Chem. 10, 439–462 (2017).
Xi, H. D. et al. Active droplet sorting in microfluidics: a review. Lab Chip 17, 751–771 (2017).
Bogojevic, D., Chamberlain, M. D., Barbulovic-Nad, I. & Wheeler, A. R. A digital microfluidic method for multiplexed cell-based apoptosis assays. Lab Chip 12, 627–634 (2012).
Tomasi, R. F., Sart, S., Champetier, T. & Baroud, C. N. Individual control and quantification of 3D spheroids in a high-density microfluidic droplet array. Cell Rep. 31, 107670 (2020).
Letai, A. Functional precision cancer medicine-moving beyond pure genomics. Nat. Med. 23, 1028–1035 (2017).
Horowitz, L. F. et al. Multiplexed drug testing of tumor slices using a microfluidic platform. NPJ Precis. Oncol. 4, 12 (2020).
Bild, A. H. et al. Oncogenic pathway signatures in human cancers as a guide to targeted therapies. Nature 439, 353–357 (2006).
Menden, M. P. et al. A cancer pharmacogenomic screen powering crowd-sourced advancement of drug combination prediction. Nat. Commun. 10, 2674 (2019).
Parca, L. et al. Modeling cancer drug response through drug-specific informative genes. Sci. Rep. 9, 15222 (2019).
Iorio, F. et al. A landscape of pharmacogenomic interactions in cancer. Cell 166, 740–754 (2016).
Bansal, M. et al. A community computational challenge to predict the activity of pairs of compounds. Nat. Biotechnol. 32, 1213–1222 (2014).
Ruppen, J. et al. Towards personalized medicine: chemosensitivity assays of patient lung cancer cell spheroids in a perfused microfluidic platform. Lab Chip 15, 3076–3085 (2015).
Astolfi, M. et al. Micro-dissected tumor tissues on chip: an ex vivo method for drug testing and personalized therapy. Lab Chip 16, 312–325 (2016).
Wong, A. H. et al. Drug screening of cancer cell lines and human primary tumors using droplet microfluidics. Sci. Rep. 7, 9109 (2017).
Lee, D. W. et al. High-throughput screening (HTS) of anticancer drug efficacy on a micropillar/microwell chip platform. Anal. Chem. 86, 535–542 (2014).
Baret, J. C. et al. Fluorescence-activated droplet sorting (FADS): efficient microfluidic cell sorting based on enzymatic activity. Lab Chip 9, 1850–1858 (2009).
Gielen, F. et al. Ultrahigh-throughput-directed enzyme evolution by absorbance-activated droplet sorting (AADS). Proc. Natl Acad. Sci. USA 113, E7383 (2016).
Schmid, L., Weitz, D. A. & Franke, T. Sorting drops and cells with acoustics: acoustic microfluidic fluorescence-activated cell sorter. Lab Chip 14, 3710–3718 (2014).
Sciambi, A. & Abate, A. R. Accurate microfluidic sorting of droplets at 30 kHz. Lab Chip 15, 47–51 (2015).
Zinchenko, A. et al. One in a million: flow cytometric sorting of single cell-lysate assays in monodisperse picolitre double emulsion droplets for directed evolution. Anal. Chem. 86, 2526–2533 (2014).
Shembekar, N., Chaipan, C., Utharala, R. & Merten, C. A. Droplet-based microfluidics in drug discovery, transcriptomics and high-throughput molecular genetics. Lab Chip 16, 1314–1331 (2016).
Shields, C. W. T., Reyes, C. D. & Lopez, G. P. Microfluidic cell sorting: a review of the advances in the separation of cells from debulking to rare cell isolation. Lab Chip 15, 1230–1249 (2015).
Carey, T. R., Cotner, K. L., Li, B. & Sohn, L. L. Developments in label-free microfluidic methods for single-cell analysis and sorting. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 11, e1529 (2019).
Tang, W. et al. Recent advances in microfluidic cell sorting techniques based on both physical and biochemical principles. Electrophoresis 40, 930–954 (2019).
Catarino, S. O. et al. Blood cells separation and sorting techniques of passive microfluidic devices: from fabrication to applications. Micromachines 10, https://doi.org/10.3390/mi10090593 (2019).
Hu, X. et al. Marker-specific sorting of rare cells using dielectrophoresis. Proc. Natl Acad. Sci. USA 102, 15757–15761 (2005).
Landry, Z. C., Giovanonni, S. J., Quake, S. R. & Blainey, P. C. Optofluidic cell selection from complex microbial communities for single-genome analysis. Methods Enzymol. 531, 61–90 (2013).
Fu, A. Y., Spence, C., Scherer, A., Arnold, F. H. & Quake, S. R. A microfabricated fluorescence-activated cell sorter. Nat. Biotechnol. 17, 1109–1111 (1999).
Dittrich, P. S. & Schwille, P. An integrated microfluidic system for reaction, high-sensitivity detection, and sorting of fluorescent cells and particles. Anal. Chem. 75, 5767–5774 (2003).
Chen, C. H. et al. Specific sorting of single bacterial cells with microfabricated fluorescence-activated cell sorting and tyramide signal amplification fluorescence in situ hybridization. Anal. Chem. 83, 7269–7275 (2011).
Chung, K., Crane, M. M. & Lu, H. Automated on-chip rapid microscopy, phenotyping and sorting of C. elegans. Nat. Methods 5, 637–643 (2008).
Zang, E. et al. Real-time image processing for label-free enrichment of Actinobacteria cultivated in picolitre droplets. Lab Chip 13, 3707–3713 (2013).
Kovac, J. R. & Voldman, J. Intuitive, image-based cell sorting using optofluidic cell sorting. Anal. Chem. 79, 9321–9330 (2007).
Kurup, G. K. & Basu, A. S. Field-free particle focusing in microfluidic plugs. Biomicrofluidics 6, 22008–2200810 (2012).
Thornberry, N. A. et al. A novel heterodimeric cysteine protease is required for interleukin-1 β processing in monocytes. Nature 356, 768–774 (1992).
Tartier, L., McCarey, Y. L., Biaglow, J. E., Kochevar, I. E. & Held, K. D. Apoptosis induced by dithiothreitol in HL-60 cells shows early activation of caspase 3 and is independent of mitochondria. Cell Death Differ. 7, 1002–1010 (2000).
Stoeckius, M. et al. Simultaneous epitope and transcriptome measurement in single cells. Nat. Methods 14, 865–868 (2017).
Zhao, B., Summers, F. A. & Mason, R. P. Photooxidation of Amplex red to resorufin: implications of exposing the Amplex red assay to light. Free Radic. Biol. Med. 53, 1080–1087 (2012).
Debski, D. et al. Mechanism of oxidative conversion of Amplex (R) red to resorufin: pulse radiolysis and enzymatic studies. Free Radic. Biol. Med. 95, 323–332 (2016).
Frenzel, D. & Merten, C. A. Microfluidic train station: highly robust and multiplexable sorting of droplets on electric rails. Lab Chip 17, 1024–1030 (2017).
Choi, K., Ng, A. H., Fobel, R. & Wheeler, A. R. Digital microfluidics. Annu. Rev. Anal. Chem. 5, 413–440 (2012).
Hess, J. F. et al. Library preparation for next generation sequencing: a review of automation strategies. Biotechnol. Adv. 41, 107537 (2020).
Gonzalez, R., Lee, J. W. & Schultz, P. G. Stepwise chemically induced cardiomyocyte specification of human embryonic stem cells. Angew. Chem. Int. Ed. 50, 11181–11185 (2011).
Wu, X., Ding, S., Ding, Q., Gray, N. S. & Schultz, P. G. Small molecules that induce cardiomyogenesis in embryonic stem cells. J. Am. Chem. Soc. 126, 1590–1591 (2004).
Han, Y. et al. Mesenchymal stem cells for regenerative medicine. Cells 8, 886 (2019).
Fang, T.-C. & Poulsom, R. Cell-based therapies for birth defects: a role for adult stem cell plasticity. Birth Defects Res. C. 69, 238–249 (2003).
Jiang, H. et al. Droplet-based light-sheet fluorescence microscopy for high-throughput sample preparation, 3-D imaging and quantitative analysis on a chip. Lab Chip 17, 2193–2197 (2017).
Woronoff, G. et al. New generation of amino coumarin methyl sulfonate-based fluorogenic substrates for amidase assays in droplet-based microfluidic applications. Anal. Chem. 83, 2852–2857 (2011).
Teh, S. Y., Lin, R., Hung, L. H. & Lee, A. P. Droplet microfluidics. Lab Chip 8, 198–220 (2008).
Rajput, A. et al. Characterization of HCT116 human colon cancer cells in an orthotopic model. J. Surg. Res. 147, 276–281 (2008).
Xia, Y. et al. Replica molding using polymeric materials: a practical step toward nanomanufacturing. Adv. Mater. 9, 147–149 (1997).
Zhang, J. H., Chung, T. D. & Oldenburg, K. R. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J. Biomol. Screen 4, 67–73 (1999).
Acknowledgements
A.G., R.U., V.V. and W.M. were supported by Germany’s Federal Ministry for Economic Affairs and Energy EXIST programme (TheraMe! project). D.T. was supported by the EMBL Interdisciplinary Postdoc Programme (EIPOD) under Marie Skłodowska-Curie COFUND Actions (grant number 291772) and by JRC-COMBINE, partially funded by Bayer. M.B. and N.T. were supported by the EMBL International PhD Programme. O.I. was supported by German Research Foundation (DFG) grant 411368829 SA 3536/2-1.
Author information
Authors and Affiliations
Contributions
C.A.M. conceived the combinatorial microfluidic platform and the Braille sorter and supervised the project with support from J.S.-R. R.U. developed the Braille valve system, the microfluidic chip designs and the LabVIEW applications and carried out the sorting experiments. V.V. performed the combinatorial drug screenings. A.G. developed the assay for drug screening with normalization to cell number. A.G., V.V., D.T., M.B. and N.P. troubleshooted and refined the microfluidics and cell culture protocols. D.T., N.P. and O.I. developed the plugy software. D.T. and V.V. analyzed the drug screening data. N.T. and W.M. produced some of the figures. A.G.O. developed and built the BD controller. All authors contributed to the writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
Parts of the technology described here are the subject of patents, including individual authors of this protocol as inventors. If the patents ever get licensed, these authors might profit financially though the inventors’ rewards programs of the involved institutes. J.S.-R. has received funding from GSK and Sanofi and consultant fees from Travere Therapeutics and Astex Pharmaceuticals.
Peer review
Peer review information
Nature Protocols thanks Chia-Hung Chen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related Links
Key references using this protocol
Eduati, F. et al. Nat. Commun. 9, 2434 (2018): https://doi.org/10.1038/s41467-018-04919-w
Utharala, R. et al. Anal. Chem. 90, 5982–5988 (2018): https://doi.org/10.1021/acs.analchem.7b04689
Mathur, L. et al. Nat. Commun. 13, 4450 (2022): https://doi.org/10.1038/s41467-022-32197-0
Extended data
Extended Data Fig. 1 Equipment used for the BD drug screening platform.
a, The main equipment used for drug screening: (1) BD, (2) Luer-lock syringes, (3) needles, (4) syringe tubing, (5) waste tubing and container, (6) cell syringe tubing, (7) PDMS chip, (8) Aquapel syringe, (9) blue color syringe, (10) WiFI microscope camera, (11) collection tube prefilled with plugs, (12) tweezers, (13) scissors, (14) wax melter and (15) wax. b, BD and custom-made BD controller. The BD is built into an aluminum frame, and a plexiglas with spring-loaded bolts holds the chip in place. c, The electronic part of BD should be protected from liquids, e.g., by covering with a rubber glove. Take care not to let it heat up excessively.
Extended Data Fig. 2 Steps for preparation of the cell syringe with tubing directly attached to the syringe without a needle.
a, Preparation based on a needle: a 23 G needle is used and the metal part is heated so that it can be removed from the plastic part. In the top of the plastic part, a hole is punched using a 0.75 mm puncher, then the cell tubing is inserted and fixed by UV glue. Finally, the syringe is autoclaved. b, Alternative method for syringe preparation based on PDMS: PDMS is mixed using elastomer and curing agent in a ratio of 1:10 in a plastic beaker, and syringes are inserted into the PDMS. The PDMS is degassed in a desiccator and cured overnight at 65 °C. Syringes are separated using a scalpel, and the cell tubing is inserted into the PDMS, so that the end of the tubing is outside the PDMS. The tube is fixed with UV glue and autoclaved. c, Setup for stirring cells: ice pack and a magnetic stirrer are placed on top of the cell syringe to ensure cooling and agitation of cells (here we used a VP710 magnetic tumble stirrer as also used in DropSeq1, but more conventional stirrers turned upside down can be used as well).
Extended Data Fig. 3 High-throughput combinatorial drug screening on human HCT-116 cells.
The experiment is exhibited in two cycles, while each combination was repeated in 12 plugs and separated from the next sample by five barcode (BC) plugs. a, Screenshot shows 12 replicates of a highly efficient drug combination (middle) compared with two low efficiency drug combinations (left and right), which are separated by 5 BCs. Caspase 3/9 signal (green) is strongly induced in highly efficient drug combinations samples, and cell number (orange) is relatively equal for all three conditions. b, Quality-control plot for the normalized caspase activity of cells in the FS controls during cycle 1 and cycle 2. c, Overview of the caspase signal normalized to the cell number for cycles 1 and 2. Each violin corresponds to one sample condition and shows the distribution of the normalized caspase signal for the 12 sample replicates. d, Heatmap representing the drug efficacy (single or in combination) in HCT-116 cells for cycle 1 and cycle 2. All conditions are normalized to a regression curve through the caspase activity of untreated cells in FS medium. The Zm value for cycle 1 = 0.49 and for cycle 2 = 0.42 (average for both cycles 0.45). e, Drug hits from both screening cycles with a mean Z score above 0.2 are listed in the table (top ten are indicated). These are single or combinatorial drugs, which significantly induced apoptosis compared with FS control medium. Positive controls are not shown, and their mean Z scores is above that of all other drugs. The complete list is available as Extended Data Table 2.
Extended Data Fig. 4 Examples of signals resulting from fused peaks or split up fragments of plugs.
a, Black arrows show reduced plug length caused by fragmentation of plugs. Fusion of two plugs is identified by increased plug length as indicated by the white arrow head. b, Two peaks resulting from plug fragmentation are indicated with black arrows.
Supplementary information
Supplementary Information
Supplementary Protocols 1–5, Fig. 1–7 and Tables 1–3.
Supplementary Video 1
Working principle of generating plugs using the BD valve system.
Supplementary Video 2
Testing Braille pin movements.
Supplementary Video 3
Alignment of screening chip on braille valves.
Supplementary Video 4
Collection, incubation and fluorescence readout of plugs.
Supplementary Software 1
All SolidWorks 3D designs of the inhouse parts for the BD platform. The easm file extension is an eDrawings Assembly file, which is a type of CAD file format in SolidWorks software.
Supplementary Software 2
Code for the Braille control board, using the BASCOM-AVR software.
Supplementary Software 3
All CAD designs of the microfluidic chips used in this protocol.
Supplementary Software 4
Plugy input data.
Supplementary Software 5
All files for manufacturing and running the PCB.
Supplementary Software 6
All LabVIEW files used in this protocol.
Supplementary Software 7
All Python files for data analysis.
Supplementary Software 8
File specifying a sequence of Braille pin actuations, used for sample barcoding. Barcoding was used to keep track of the combinations that are being generated. Each series of 12 replicates of a treatment condition is followed by 5 barcode plugs (Cascade blue- a blue fluorescent dye).
Supplementary Software 9
File specifying which drug is connected to which inlet of the Braille valve chip (needed for data analysis).
Supplementary Software 10
File specifying a sequence of Braille pin actuations, used for chip testing. The column contains the opening time of the valve, the number of repeats, sample name and 4 numbers of valves, which are opened at the same time.
Supplementary Software 11
File specifying a sequence of Braille pin actuation, used for priming the setup prior to a drug screen. (For more details please see Box 1.).
Supplementary Software 12
File specifying a sequence of Braille pin actuations, used for drug screening. It contains information on which pins are actuated and also the exact timing.
Source data
Source Data Fig. 4
Numeric raw data for the screen illustrated in Fig. 4d,e.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Utharala, R., Grab, A., Vafaizadeh, V. et al. A microfluidic Braille valve platform for on-demand production, combinatorial screening and sorting of chemically distinct droplets. Nat Protoc 17, 2920–2965 (2022). https://doi.org/10.1038/s41596-022-00740-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-022-00740-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.