Abstract
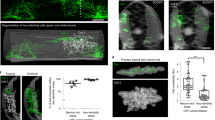
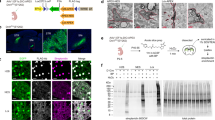
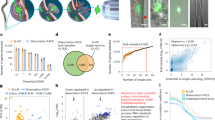
During neuronal development, growth cones (GCs) of projection neurons navigate complex extracellular environments to reach distant targets, thereby generating extraordinarily complex circuitry. These dynamic structures located at the tips of axonal projections respond to substrate-bound as well as diffusible guidance cues in a neuronal subtype– and stage-specific manner to construct highly specific and functional circuitry. In vitro studies of the past decade indicate that subcellular localization of specific molecular machinery in GCs underlies the precise navigational control that occurs during circuit ‘wiring’. Our laboratory has recently developed integrated experimental and analytical approaches enabling high-depth, quantitative proteomic and transcriptomic investigation of subtype- and stage-specific GC molecular machinery directly from the rodent central nervous system (CNS) in vivo. By using these approaches, a pure population of GCs and paired somata can be isolated from any neuronal subtype of the CNS that can be fluorescently labeled. GCs are dissociated from parent axons using fluid shear forces, and a bulk GC fraction is isolated by buoyancy ultracentrifugation. Subtype-specific GCs and somata are purified by recently developed fluorescent small particle sorting and established FACS of neurons and are suitable for downstream analyses of proteins and RNAs, including small RNAs. The isolation of subtype-specific GCs and parent somata takes ~3 h, plus sorting time, and ~1–2 h for subsequent extraction of molecular contents. RNA library preparation and sequencing can take several days to weeks, depending on the turnaround time of the core facility involved.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
Most data included were first described in our earlier paper13, and the figure legends describe specifically where each piece of data included was published previously. Examples of datasets generated and analyzed by using the presented approaches are also available in the Harvard Dataverse repository (https://doi.org/10.7910/DVN/ISOEB6 (ref. 13).
References
Cajal, S. R. A quelle époque apparaissent les expansions des cellules nerveuses de la moëlle épinière du poulet?. Anat. Anz. 5, 609–613, 621–631 (1890).
Menon, S. & Gupton, S. L. Building blocks of functioning brain: cytoskeletal dynamics in neuronal development. Int. Rev. Cell Mol. Biol. 322, 183–245 (2016).
Tamariz, E. & Varela-Echavarria, A. The discovery of the growth cone and its influence on the study of axon guidance. Front. Neuroanat. 9, 51 (2015).
Vitriol, E. A. & Zheng, J. Q. Growth cone travel in space and time: the cellular ensemble of cytoskeleton, adhesion, and membrane. Neuron 73, 1068–1081 (2012).
Ye, X., Qiu, Y., Gao, Y., Wan, D. & Zhu, H. A subtle network mediating axon guidance: intrinsic dynamic structure of growth cone, attractive and repulsive molecular cues, and the intermediate role of signaling pathways. Neural Plast. 2019, 1719829 (2019).
Dent, E. W., Gupton, S. L. & Gertler, F. B. The growth cone cytoskeleton in axon outgrowth and guidance. Cold Spring Harb. Perspect. Biol. 3, a001800 (2011).
Bellon, A. & Mann, F. Keeping up with advances in axon guidance. Curr. Opin. Neurobiol. 53, 183–191 (2018).
Kaplan, A., Kent, C. B., Charron, F. & Fournier, A. E. Switching responses: spatial and temporal regulators of axon guidance. Mol. Neurobiol. 49, 1077–1086 (2014).
Stoeckli, E. T. Understanding axon guidance: are we nearly there yet? Development 145, dev151415 (2018).
Costa, C. J. & Willis, D. E. To the end of the line: axonal mRNA transport and local translation in health and neurodegenerative disease. Dev. Neurobiol. 78, 209–220 (2018).
Holt, C. E. & Schuman, E. M. The central dogma decentralized: new perspectives on RNA function and local translation in neurons. Neuron 80, 648–657 (2013).
Sasaki, Y. Local translation in growth cones and presynapses, two axonal compartments for local neuronal functions. Biomolecules 10, 668 (2020).
Poulopoulos, A. et al. Subcellular transcriptomes and proteomes of developing axon projections in the cerebral cortex. Nature 565, 356–360 (2019).
Lohse, K. et al. Axonal origin and purity of growth cones isolated from fetal rat brain. Brain Res. Dev. Brain Res. 96, 83–96 (1996).
Pfenninger, K. H., Ellis, L., Johnson, M. P., Friedman, L. B. & Somlo, S. Nerve growth cones isolated from fetal rat brain: subcellular fractionation and characterization. Cell 35, 573–584 (1983).
Catapano, L. A., Arnold, M. W., Perez, F. A. & Macklis, J. D. Specific neurotrophic factors support the survival of cortical projection neurons at distinct stages of development. J. Neurosci. 21, 8863–8872 (2001).
Ellis, L., Katz, F. & Pfenninger, K. H. Nerve growth cones isolated from fetal rat brain. II. Cyclic adenosine 3′:5′-monophosphate (cAMP)-binding proteins and cAMP-dependent protein phosphorylation. J. Neurosci. 5, 1393–1401 (1985).
Ellis, L., Wallis, I., Abreu, E. & Pfenninger, K. H. Nerve growth cones isolated from fetal rat brain. IV. Preparation of a membrane subfraction and identification of a membrane glycoprotein expressed on sprouting neurons. J. Cell Biol. 101, 1977–1989 (1985).
Katz, F., Ellis, L. & Pfenninger, K. H. Nerve growth cones isolated from fetal rat brain. III. Calcium-dependent protein phosphorylation. J. Neurosci. 5, 1402–1411 (1985).
Brittis, P. A., Lu, Q. & Flanagan, J. G. Axonal protein synthesis provides a mechanism for localized regulation at an intermediate target. Cell 110, 223–235 (2002).
Farina, K. L., Huttelmaier, S., Musunuru, K., Darnell, R. & Singer, R. H. Two ZBP1 KH domains facilitate beta-actin mRNA localization, granule formation, and cytoskeletal attachment. J. Cell Biol. 160, 77–87 (2003).
Gumy, L. F. et al. Transcriptome analysis of embryonic and adult sensory axons reveals changes in mRNA repertoire localization. RNA 17, 85–98 (2011).
Leung, K. M. et al. Asymmetrical β-actin mRNA translation in growth cones mediates attractive turning to netrin-1. Nat. Neurosci. 9, 1247–1256 (2006).
Leung, L. C. et al. Coupling of NF-protocadherin signaling to axon guidance by cue-induced translation. Nat. Neurosci. 16, 166–173 (2013).
Minis, A. et al. Subcellular transcriptomics-dissection of the mRNA composition in the axonal compartment of sensory neurons. Dev. Neurobiol. 74, 365–381 (2014).
Taylor, A. M. et al. Axonal mRNA in uninjured and regenerating cortical mammalian axons. J. Neurosci. 29, 4697–4707 (2009).
Wu, K. Y. et al. Local translation of RhoA regulates growth cone collapse. Nature 436, 1020–1024 (2005).
Yoon, B. C. et al. Local translation of extranuclear lamin B promotes axon maintenance. Cell 148, 752–764 (2012).
Zivraj, K. H. et al. Subcellular profiling reveals distinct and developmentally regulated repertoire of growth cone mRNAs. J. Neurosci. 30, 15464–15478 (2010).
Arlotta, P. et al. Neuronal subtype-specific genes that control corticospinal motor neuron development in vivo. Neuron 45, 207–221 (2005).
Clare, A. J., Day, R. C., Empson, R. M. & Hughes, S. M. Transcriptome profiling of layer 5 intratelencephalic projection neurons from the mature mouse motor cortex. Front. Mol. Neurosci. 11, 410 (2018).
Kawasawa, Y. I. et al. RNA-seq analysis of developing olfactory bulb projection neurons. Mol. Cell. Neurosci. 74, 78–86 (2016).
Molyneaux, B. J. et al. Novel subtype-specific genes identify distinct subpopulations of callosal projection neurons. J. Neurosci. 29, 12343–12354 (2009).
Cagnetta, R., Frese, C. K., Shigeoka, T., Krijgsveld, J. & Holt, C. E. Rapid cue-specific remodeling of the nascent axonal proteome. Neuron 99, 29–46.e4 (2018).
Shigeoka, T. et al. Dynamic axonal translation in developing and mature visual circuits. Cell 166, 181–192 (2016).
Saito, T. & Nakatsuji, N. Efficient gene transfer into the embryonic mouse brain using in vivo electroporation. Dev. Biol. 240, 237–246 (2001).
Trichas, G., Begbie, J. & Srinivas, S. Use of the viral 2A peptide for bicistronic expression in transgenic mice. BMC Biol. 6, 40 (2008).
Filipe, V., Hawe, A. & Jiskoot, W. Critical evaluation of Nanoparticle Tracking Analysis (NTA) by NanoSight for the measurement of nanoparticles and protein aggregates. Pharm. Res. 27, 796–810 (2010).
Wang, L., Wang, S. & Li, W. RSeQC: quality control of RNA-seq experiments. Bioinformatics 28, 2184–2185 (2012).
Hatch, J., Poulopoulos, A., Engmann, A. K. & Macklis, J. D. Growth cone molecular machinery locally implements the development of subtype-specific neocortical circuitry. Soc. Neurosci. Abstr. 365, 11 (2019).
Spandidos, A., Wang, X., Wang, H. & Seed, B. PrimerBank: a resource of human and mouse PCR primer pairs for gene expression detection and quantification. Nucleic Acids Res. 38, D792–D799 (2010).
Cox, J. et al. Accurate proteome-wide label-free quantification by delayed normalization and maximal peptide ratio extraction, termed MaxLFQ. Mol. Cell. Proteom. 13, 2513–2526 (2014).
Cox, J. & Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 26, 1367–1372 (2008).
Tyanova, S. et al. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods 13, 731–740 (2016).
Zhang, X. et al. Proteome-wide identification of ubiquitin interactions using UbIA-MS. Nat. Protoc. 13, 530–550 (2018).
Bray, N. L., Pimentel, H., Melsted, P. & Pachter, L. Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol. 34, 525–527 (2016).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Li, B. & Dewey, C. N. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics 12, 323 (2011).
Acknowledgements
We dedicate this paper to the memory of John J. Hatch, who believed deeply in sharing resources, expertise, data and his excitement and exceptional abilities to enable others to deeply, rigorously and creatively investigate the subcellular biology of polarized cells, developing neurons and circuits in particular. We thank our laboratory colleague Dustin Tillman for a representative image of the hydrolysis protection assay. We thank Joyce LaVecchio and Nema Kheradmand of the HSCRB-HSCI Flow Cytometry Core; Emma White, Christian Daly and Claire Hartmann of the Harvard Bauer Sequencing Core; William Lane, John Neveu and Bogdan Budnik of the Harvard FAS CSB Mass Spectrometry and Proteomics Resource Lab; Anna-Katerina Hadjantonakis (Memorial Sloan Kettering) and Shankar Srinivas (Oxford) for reagents. Analytic tools and infrastructure in the Harvard Chan Bioinformatics Core were partially supported by funds from the Harvard NeuroDiscovery Center and the Harvard Stem Cell Institute. This work was supported by the following grants to J.D.M.: NIH Pioneer Award DP1NS106665; Paul G. Allen Frontiers Group—Allen Distinguished Investigator award and Brain Research Foundation Scientific Innovations Award; Max and Anne Wien Professor of Life Sciences fund; Emily and Robert Pearlstein Fund; and additional infrastructure support from NIH grants NS045523, NS104055, NS075672 and NS049553. J.J.H. was partially supported by NIH individual Training Grant F31 NS103262. A.K.E. was partially supported by the Jean-Jacques & Felicia Lopez-Loreta Foundation, the Travis Roy Foundation (to J.D.M.) and a Swiss National Science Foundation Fellowship. A.P. was partially supported by a European Molecular Biology Long-Term Fellowship and a Human Frontiers Science Program Long-Term Fellowship. A.J.M. was partially supported by NIH Training Grant T32 AG000222.
Author information
Authors and Affiliations
Contributions
A.K.E., J.J.H., A.P., P.V. and J.D.M. wrote the manuscript. A.P., A.J.M., J.J.H., A.O. and A.K.E. performed experiments and analyzed data. A.P., J.J.H., P.N. and A.K.E. designed figures.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Protocols thanks Michael Sendtner and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key reference using this protocol
Poulopoulos, A. et al. Nature 565, 356–360 (2019) https://doi.org/10.1038/s41586-018-0847-y
Supplementary information
Supplementary Information
Supplementary Fig. 1.
Rights and permissions
About this article
Cite this article
Engmann, A.K., Hatch, J.J., Nanda, P. et al. Neuronal subtype-specific growth cone and soma purification from mammalian CNS via fractionation and fluorescent sorting for subcellular analyses and spatial mapping of local transcriptomes and proteomes. Nat Protoc 17, 222–251 (2022). https://doi.org/10.1038/s41596-021-00638-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-021-00638-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.