Abstract
Clinically available imaging tools for diagnosing infections rely on structural changes in the affected tissues. They therefore lack specificity and cannot differentiate between oncologic, inflammatory and infectious processes. We have developed 2-deoxy-2-[18F]fluoro-d-sorbitol (18F-FDS) as an imaging agent to visualize infections caused by Enterobacterales, which represent the largest group of bacterial pathogens in humans and are responsible for severe infections, often resulting in sepsis or death. A clinical study in 26 prospectively enrolled patients demonstrated that 18F-FDS positron emission tomography (PET) was safe, and could detect and localize infections due to drug-susceptible or multi-drug-resistant Enterobacterales strains as well as differentiate them from other pathologies (sterile inflammation or cancer). 18F-FDS is cleared almost exclusively through renal filtration and has also shown potential as a PET agent for functional renal imaging. Since most PET radionuclides have a short half-life, maximal clinical impact will require fast, on-demand synthesis with limited infrastructure and personnel. To meet this demand, we developed a kit-based solid phase method that uses commercially and widely available 2-deoxy-2-[18F]fluoro-d-glucose as the precursor and allows 18F-FDS to be produced and purified in one step at room temperature. The 18F-FDS kit consists of a solid-phase extraction cartridge packed with solid supported borohydride (MP-borohydride), which can be attached to a second cartridge to reduce pH. We evaluated the effects of different solid supported borohydride reagents, cartridge size, starting radioactivity, volumes and flow rates in the radiochemical yield and purity. The optimized protocol can be completed in <30 min and allows the synthesis of 18F-FDS in >70% radiochemical yield and >90% radiochemical purity.
Similar content being viewed by others
Introduction
Positron emission tomography (PET) is a noninvasive imaging technology that utilizes radiotracers to visualize molecular biology in situ. PET relies on localizing scintillation events due to positron decay of radioisotopes bound to a target molecule of interest. PET is now available as a routine clinical tool, particularly for oncology, neurology and cardiology1, in developed and developing countries2. PET imaging typically takes 15–60 min and is usually performed 1–2 h after intravenous administration of the radiotracer. Recent developments such as total-body PET have shortened the scan duration and increased sensitivity3.
The incorporation of positron-emitting radionuclides into appropriate tracer molecules takes place in radiochemistry facilities with appropriate radiation protection amenities and by qualified personnel. Therefore, clinical adoption of new PET tracers depends on the development of fast, simple and reproducible radiolabeling strategies. Automated radiosyntheses are preferred as they reduce user-bias and radiation exposure to the radiochemist. However, these are often limited to facilities with access to an in-house cyclotron, and the International Atomic Energy Agency estimates a total of ~6,000 PET scanners and ~1,000 cyclotrons worldwide4. Nuclear imaging centers with PET scanners but without cyclotrons rely on radiotracers being synthesized off-site. Due to radioactive decay, the transportation of PET tracers from the production site to the clinical PET scanner must be achieved within hours, being often limited to fluorine-18-labeled radiotracers (18F, t1/2 = 110 min). Conversely, radiometals (e.g., 99mTc, 111In, 67/68Ga) used in PET and single-photon emission computed tomography imaging are commonly incorporated into molecules by simple one-step kit-based mechanisms on site, without the need for specialized radiosynthesis and purification facilities.
Recently, advancements in late-stage fluorination mechanisms and solid-supported syntheses have enable the application of one-step kit-based synthesis to 18F-labeled PET tracers, which, once developed, require minimal technical skills5,6,7,8,9,10.
2-Deoxy-2-[18F]fluoro-d-sorbitol (18F-FDS), a clinical PET tracer for bacterial imaging
Enterobacterales, such as Escherichia coli, Klebsiella pneumoniae, Enterobacter spp., Salmonella spp., Serratia spp. and Yersinia spp., are Gram-negative bacteria representing the largest group of bacterial pathogens in humans11. They produce a range of severe infections that can result in sepsis or death. Multi-drug-resistant (MDR) strains have become widespread globally and thus designated as urgent threats to human health12. Importantly, antimicrobial drug resistance is among the top ten threats to human health, and it is estimated that drug-resistant infections will become the leading cause of death globally, surpassing those due to cancer by 205013. A cumulative 100 trillion USD of economic output is at risk due to the rise of drug-resistant infections.
Diagnosis of bacterial infections requires the isolation of the pathogen in available clinical samples such as blood, urine, stool or cerebrospinal fluid. However, these clinical samples are often nondiagnostic or insensitive for the detection of deep-seated bacterial infections14. Surgical resection or biopsy of infected tissues is often considered the last resort for establishing a definitive diagnosis in such situations, but sampling bias and accessibility hinder and limit these approaches. Finally, clinically available imaging tools such as radiography, ultrasonography, computed tomography (CT), and magnetic resonance imaging rely on structural changes in anatomy or tissue morphology that are often delayed relative to the disease process, are nonspecific, and reflect a combination of the infection and the host inflammatory response. Therefore, there is an unmet clinical need for rapid, whole-body and specific imaging methods to detect bacteria in situ. Noninvasive molecular imaging technologies such as PET can overcome these limitations by employing radiolabeled molecules that selectively accumulate in bacteria to visualize and localize infections in the body with high specificity15,16.
Pathogen-specific imaging relies on metabolic, structural or mechanistic differences between mammalian and bacterial cells. Radiotracers under development for bacterial imaging include small molecules targeting carbohydrate metabolism17,18, bacterial folate biosynthesis19,20,21,22, iron transport23, d-amino acids24 and antimicrobial peptides25, which have been discussed in recent reviews15,26. Targeting metabolic pathways offers the advantage of intracellular enzymatic turnover and trapping, leading to accumulation and signal amplification over background signal from unaffected mammalian tissues. 2-Deoxy-2-[18F]fluoro-d-sorbitol (18F-FDS) is a sugar alcohol that selectively accumulates in the Enterobacterales class of bacteria via a metabolically conserved sorbitol-specific pathway.
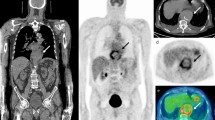
In mouse models, 18F-FDS PET/CT was able to distinguish infection from sterile inflammation and detect therapeutic failures associated with MDR, extended-spectrum β-lactamase-producing E. coli strains18,27,28. In a hamster model of COVID-19, 18F-FDS PET/CT selectively differentiated severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pneumonia from secondary Klebsiella pneumoniae pneumonia; i.e., 18F-FDS was not taken up in the lungs of hamsters infected only with SARS-CoV-216. In a prospective clinical study, 18F-FDS PET/CT successfully detected Enterobacterales infections due to drug-susceptible or MDR strains and distinguished them from sterile inflammation or cancerous lesions. Repeat imaging after antibiotic treatments revealed that 18F-FDS was also able to monitor antimicrobial response (Fig. 1)16.
a, Three-dimensional maximum intensity projection (MIP) from a patient with microbiologically confirmed Enterobacter aerogenes cellulitis of the left breast. Signal is also noted in the heart (blood pool), liver, kidneys and the urinary bladder. b, Three-dimensional MIP from a patient with MDR, extended spectrum beta-lactamase (ESBL)-producing E. coli osteomyelitis before and after inadequate treatment. Yellow arrows indicate site of infection. Images adapted with permission from ref. 16. Copyright 2021 The American Association for the Advancement of Science.
Limitations
There are some limitations to this technology. 18F-FDS PET can specifically detect infections due to Enterobacterales but not all classes of bacteria. However, it should be noted that Enterobacterales represent the largest group of bacterial pathogens in humans11, and are frequently associated with MDR infections, which are designated as urgent threats to human health12. Additionally, 18F-FDS requires bacterial enzymatic activity based on adenosine triphosphate and so theoretically may not fare well in clinical infections where the predominant bacterial populations are metabolically inactive. Finally, PET is not widely available, and when a patient presents with a suspected infection of unknown origin, maximum benefit from diagnostic PET will be achieved only if the patient can be imaged on the same day, before starting empiric antibiotic therapy. Successful implementation of routine PET studies for diagnostic and treatment selection purposes will therefore benefit greatly from on-demand availability of pathogen-specific PET tracers29.
18F-FDS, a clinical PET tracer for functional renal imaging
Molecular imaging to evaluate renal function is an evolving field30. Current approaches are focused on technetium-99m radiopharmaceuticals (e.g., 99mTc-mercaptoacetyltriglycine and 99mTc-diethylenetriaminepentaacetic acid) combined with planar gamma cameras. Although these techniques are widely used in the clinic, they are limited by two-dimensional information leading to a lack of accurate anatomic correlation, low spatial resolution and low signal/background ratios. Soft-tissue attenuation associated with mostly low-energy radionuclides may also limit reliable quantification31. Therefore, there has been increasing interest in developing PET-based functional renal imaging agents, which offer advantages such as three-dimensional dynamic visualization of the kidneys, higher sensitivity and signal/background ratio, and absolute camera-based quantification32.
Since mammalian cells do not have mechanisms of uptake for 18F-FDS and it is predominantly cleared through the kidneys16,18,33, 18F-FDS PET has also been used as an investigational tracer for functional renal imaging34,35. Dynamic 18F-FDS PET demonstrated rapid accumulation of 18F-FDS in the renal cortex in healthy control rats. However, renal uptake of 18F-FDS was substantially delayed in rats with acute renal failure or unilateral ureteral obstruction kidneys34. Urine concentrations of 18F-FDS and 99mTc-diethylenetriaminepentaacetic acid correlated well with each other. Additionally, dynamic 18F-FDS PET performed in two healthy volunteers demonstrated rapid 18F-FDS accumulation in the renal cortex with gradual transition to the parenchyma. Given the higher spatiotemporal resolution of PET relative to conventional scintigraphy, 18F-FDS PET offers a more thorough evaluation of human renal kinetics35.
Radiosynthesis approaches for 18F-FDS
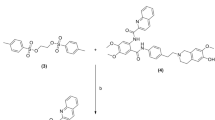
One of the main advantages of 18F-FDS is that it can be synthesized from 2-deoxy-2-[18F]fluoro-d-glucose (18F-FDG), the most widely used and commercially available PET tracer worldwide. The first reported synthesis of 18F-FDS consisted of a reduction of 18F-FDG with sodium borohydride (NaBH4) in solution (in a temperature-controlled system), followed by quenching, pH adjustment and filtering36. Since the conversion is nearly quantitative, no further purification is required. Alternative synthetic approaches have been developed, including an automated synthesis of 18F-FDS from cyclotron-generated aqueous 18F-fluoride and a solid-phase synthesis using NaBH4 on aluminum oxide (Table 1)28,37. A limitation of the automated radio-synthetic approach is that, in clinical practice, on-demand availability would not be possible as it would require constant availability of highly specialized equipment and qualified personal. Conversely, commercially available 18F-FDG can be obtained daily in most PET imaging facilities. This prompted us to develop a simple, rapid, one-step kit for on-demand synthesis of 18F-FDS from 18F-FDG.
Experimental design
We designed a self-contained kit that can be used to synthesize, purify and formulate 18F-FDS in <30 min (Fig. 2). Note that a preformulated kit would not require cartridge assembly and would therefore allow this process to be completed within 10 min. The kit uses clinical doses of 18F-FDG (max. 925 MBq) as the starting material and yields clinical doses of 18F-FDS (min. 370 MBq), which will facilitate its clinical translation. The chemical process is optimized for room temperature (20–25 °C) synthesis and, due to its simplicity, does not require any other specialized equipment. The kit consists of a small cartridge filled with a solid-supported borohydride source.
a, 18F-FDG is reduced to 18F-FDS by reacting with MP-borohydride at room temperature. b, Schematics of the in-house developed borohydride cartridge and commercially available cation exchange cartridge (PS-H+). c, Borohydride and cation-exchange cartridges are pre-equilibrated with deionized water and connected in tandem with the borohydride cartridge on top. A solution of 18F-FDG is passed through the cartridges, followed by saline, to generate 18F-FDS.
We selected macroporous triethylammonium methylpolystyrene borohydride (MP-borohydride) as the reagent for the initial prototype. In brief, MP-borohydride is a positively charged macroporous material with bound negatively charged borohydride. To determine if MP-borohydride could reduce 18F-FDG to 18F-FDS, this was first attempted using a molar equivalent of NaBH4 using the standard reduction conditions. In solution, complete reduction of 18F-FDG was achieved in 30 min, both at 35 °C and room temperature. The following experimental studies included investigating the appropriate amount of reagent that will produce high radiochemical yield (RCY) and purity, and finding the optimal volume of the starting 18F-FDG solution, and time of elution. The cartridge protocol was then optimized for use with clinical doses of 18F-FDS, and a second cartridge was added to obtain a sample pH suitable for in vivo intravenous administration. Table 2 summarizes the reaction conditions tested to optimize the protocol.
Cartridge size and reagent amount
Polymer-supported borohydrides can be used in molar excess to drive the reaction to completion more easily and decrease the reaction time. Using a closed cartridge system, there is no need for additional filtration and workups. Solid-phase cartridges were prepared with varying amounts of MP-borohydride (Table 2). Cartridges containing 300 mg of MP-borohydride were able to produce 18F-FDS with >90 % radiochemical purity (n = 8) when starting with <370 MBq of 18F-FDG. However, the radiochemical purity was reduced when using higher doses. A larger cartridge containing 500 mg of reagent was found suitable to produce 18F-FDS in >70% yield and >90% radiochemical purity when starting with up to 925 MBq (n = 6). 18F-FDS injections in humans are performed with a minimum dose of 370 MBq. Thus, we optimized the kit synthesis to start with 925 MBq of 18F-FDG, which is the maximum single dose commercially available.
Volume and elution rates
While testing different reaction conditions, we observed that preconditioning the borohydride cartridge was imperative to the synthesis. Additionally, the flow rate and volume of the 18F-FDG solution passing through the cartridge was also an important factor to consider. When the 18F-FDG solution was <1 mL and the flow rate was 1 mL/min, the RCY and radiochemical purity were lower compared with a volume >2 mL and flow rate of 0.25 mL/min (Table 2). Diluting the starting 18F-FDG solution may be required, and decreasing the flow rate allows the solution to interact longer with the borohydride, thus increasing the reducing capability of the reagent.
Alterative reagents
We tested this protocol using additional sources of solid-supported borohydride. Cartridges containing NaBH4 on aluminum oxide produced 18F-FDS with <10 % radiochemical purity under different reaction conditions (n = 4). (Polystyrylmethyl)trimethylammonium borohydride and borohydride on Amberlite IRA-400 both produced 18F-FDS with <80 % radiochemical purity (n = 3 and n = 4, respectively). MP-borohydride was superior in consistently producing high RCY and radiochemical purity >90 %.
Formulation for in vivo administration
The chemical reduction of 18F-FDG into 18F-FDS results in an increase in pH, which, when using high doses, led to pH 10. To minimize handling as well as ensure a closed kit system, we investigated if a second cartridge could be used to ensure acceptable pH for intravenous administration into animals and humans (pH 6–8). Sep-Pak alumina did not affect the pH of the sample. A chromabond SET V cartridge was found to reduce pH to 6, consistently. When starting with 857 ± 80 MBq of 18F-FDG, 523 ± 68 MBq of 18F-FDS was eluted, resulting in >60% RCY, with >90% radiochemical purity (n = 3). However, the use of this cartridge substantially increases the volume of the product, which may limit its application for preclinical studies. We found that a solid-phase extraction (SPE) cartridge packed with polystyrene-divinylbenzene copolymer with a strong cation exchanger (Chromafix-PS-H+) can be attached in line with the MP-borohydride cartridge and reduce pH from 12 to 6, without increasing volume.
Quality control
The quality control (QC) employed for our preclinical studies with kit-generated 18F-FDS consisted of visual inspection of appearance, pH measurement, and confirmation of chemical identity and purity using thin-layer chromatography (TLC) methods. Radio-TLC can be used to measure the retention factor (Rf) of 18F-FDS in silica plates, which can then be matched to the Rf of the non-radioactive standard material (FDS) visible by staining with permanganate solution, as previously reported36,38. 18F-FDS shows an Rf distinct from 18F-FDG and 18F-fluoride ion, so the same method can be used to measure radiochemical purity.
Clinical translation
The kit was designed to facilitate synthesis of 18F-FDS in PET centers where sophisticated radiochemistry facilities may not be available. As commercially available 18F-FDG synthesized under current good manufacture practices is the starting material, the number of tests required for QC is substantially reduced. For example, this method does not require the use of organic solvents or additional reagents such as phase-transfer catalysts, normally required for the syntheses of 18F tracers. Residual solvent and reagent analysis is therefore not required. However, the SPE cartridge is versatile and can be incorporated as the final step in the synthesis of 18F-FDS from in-house cyclotron-generated 18F with 18F-FDG as the intermediate. Additionally, SPE cartridges can be added to synthesis cassettes used in automated synthesis systems. While the kit method is robust and can easily be adopted by multiple centers, clinical use and method variations may require additional toxicity tests, release criteria and QC tests based on local regulations.
Materials
Reagents
Caution
When handling radioactive materials (18F-fluoride), it is important to minimize time of exposure, and ensure appropriate distance and lead shielding. Personal radiation dosimeters should be worn, and radiation exposure monitored by appropriate radiation safety authorities.
-
2-Deoxy-2-fluoro-d-glucose (FDG; Sigma-Aldrich, cat. no. F5006)
-
NaBH4 (Sigma-Aldrich, cat. no. 71320)
-
Acetic acid (Sigma-Aldrich, cat. no. A6283)
-
Sodium bicarbonate (NaHCO3; Sigma-Aldrich, cat. no. S8875)
-
Potassium permanganate (KMnO4; Sigma-Aldrich, cat. no. 223468)
-
Potassium carbonate, anhydrous (K2CO3; Sigma-Aldrich, cat. no. 791776)
-
Sodium hydroxide solution (Sigma-Aldrich, cat. no. 415413)
-
2-Deoxy-2-[18F]-fluoro-d-glucose (18F-FDG; Sofie Co (USA), or alternative source)
-
MP-borohydride (Biotage, cat. no. 801469)
-
NaBH4 on aluminum oxide (Sigma-Aldrich, cat. no. 243620)
-
(Polystyrylmethyl)trimethylammonium borohydride (Sigma-Aldrich, cat. no. 359947)
-
Borohydride on Amberlite IRA-400 (Sigma-Aldrich, cat. no. 328642)
-
Phosphate-buffered saline (PBS, 0.01 M potassium phosphate monobasic, 0.155 M sodium chloride, 0.003 M sodium phosphate dibasic, pH 7.4; Thermofisher, cat. no. 10010049)
-
Deionized water (obtained from Milli-Q integral water purification system)
-
Silica Gel 60 F254 coated aluminum-backed TLC sheets (Fisher Scientific, cat. no. M1055340001)
-
pH test strips, pH 0–14, resolution 1.0 pH unit (Sigma-Aldrich, cat. no. P4786-100EA)
Reagent setup
2-Deoxy-2-fluoro-d-sorbitol
Transfer 12 mg of 2-deoxy-2-fluoro-d-glucose to a vial, and dissolve in 1 mL of water. Add 10 mg of NaBH4, and react for 15 min at 55 °C. Upon cooling, quench with 25% acetic acid (25 µL) and adjust to pH 7 with NaHCO3. Filter through an Alumina-N Sep-Pak cartridge, and lyophilize the filtrate. 1H NMR (500 MHz, D2O) δ 4.64 − 4.36 (m, 1H), 3.95 − 3.82 (m, 1H), 3.82 − 3.60 (m, 4H), 3.60 − 3.44 (m, 2H). It can be stored in a cool dry area.
KMnO4 solution
Dissolve 1.5 g of KMnO4 and 10 g of K2CO3 in 200 mL of water. Add 1.25 mL of a 10% NaOH solution. This solution can be stored at room temperature, but should be used within 1 month of preparation.
Equipment
-
Reversible SPE tubes, 2 mL, non-fluorous polypropylene, with frit (20 μm porosity), and female Luer fitting top (Sigma-Aldrich, cat. no. 57608-U)
-
Reversible SPE tubes, 1 mL, non-fluorous polypropylene, with frit (20 μm porosity), and female Luer fitting top (Sigma-Aldrich, cat. no. 57607-U)
-
CHROMAFIX PS-H+ small SPE cartridge, 0.4 mL, polyethylene filter (Macherey-Nagel, cat. no. 731867)
-
Chromabond Set V (ABX advanced biochemical compounds, cat. no. 00260165)
-
Vials, screw top (Sigma-Aldrich, cat. No. Z115150-12A)
-
Single-use plastic syringe, 5 mL, slip-tip (BD, cat. no. 309647)
-
Needles, 21 gauge × 1.5 inches (BD, cat. no. 305167)
-
Pro-Tec PET syringe shields with lead glass window, 10 cc (Biodex, cat. no. 007-980)
-
Radio-TLC imaging scanner (Eckert + Ziegler AR-2000, or equivalent)
-
Dose calibrator (Capintec CRC-55tR, or equivalent)
Equipment setup
Cartridge preparation
-
1
Insert a 20 μm frit at the bottom of a 2 mL SPE cartridge.
-
2
Weigh out 500 mg of MP-borohydride, and transfer to the 2 mL SPE cartridge
Critical step
Other sources of borohydride have shown inferior reducing capacity (Table 2, conditions 16–24).
-
3
Add a second 20 μm frit on top of the MP-borohydride, but do not pack the frit tightly
Critical
The borohydride resin expands upon preconditioning; therefore, space between the frit and borohydride is needed (~2 mm).
-
4
Seal the top of the cartridge with a female Luer top.
Procedure
Caution
Only trained personnel should handle radioactive materials. All waste materials should be handled as radioactive waste following local radiation protection guidelines.
Cartridge pre-equilibration
Timing 3 min
-
1
Pass 2 mL of water through the MP-borohydride cartridge
Critical step
Use a moderate flow of 2 mL/min, eluting slowly and monitoring the flow with a timer. Keep the cartridge vertical, and do not dry.
-
2
Prepare the cartridge that will be used to correct the pH after synthesis. There are two options: the PS-H+ and the Chromabond Set V.
PS-H+ cartridge (for lower product volumes; recommended for small animal preclinical work)
Pass 1 mL of water through the PS-H+ cartridge
Chromabond Set V cartridge (for removal of other potential impurities; recommended if synthesizing 18F-FDG in house or for clinical use)
Pass 10 mL of water through the Chromabond Set V cartridge
18F-FDG preparation
Timing 2 min
-
3
If needed, dilute 18F-FDG solution (maximum 925 MBq (25 mCi) of activity) with water, to ensure volume is between 2 and 3 mL. Retain ~10 μL for TLC analysis (optional).
Critical step
Volumes <2 mL may result in lower RCY, and larger volumes have not been tested.
-
4
Take up the solution in a 5 mL syringe.
-
5
Measure the starting activity and time in a dose calibrator.
-
6
Place the 5 mL syringe containing diluted 18F-FDG solution in a lead syringe shield.
18F-FDG reduction to 18F-FDS
Timing 5–10 min
-
7
Place receiving vial inside a lead container (pot).
-
8
Attach 18F-FDG syringe on top of the MP-borohydride cartridge. Place above the receiving vial and clamp.
-
9
Elute 18F-FDG solution through the MP-borohydride cartridge dropwise, by pushing the plunger in the syringe.
Critical step
A slow elution yields higher purity; elution rate of ~0.3 mL/min is recommended.
-
10
Remove 18F-FDG syringe, and replace with a 5 mL syringe containing 2 mL of PBS.
-
11
Elute PBS dropwise over 2 min.
-
12
Measure pH using pH strips. This is usually pH 8–10.
-
13
Measure the activity of 18F-FDS eluted and time.
18F-FDS purification and formulation
Timing 5 min
-
14
For in vivo work, a second cartridge can be used to reduce pH and remove any potential impurities. As mentioned in Step 2, there are two options: the PS-H+ cartridge (option A) and the chromabond Set V (option B)
-
(A)
PS-H+ cartridge
-
(i)
Cartridge can be connected in line with the borohydride cartridge.
-
(ii)
Follow steps described in ‘18F-FDG reduction to 18F-FDS’.
-
(i)
-
(B)
Chromabond Set V
-
(i)
Cartridge can be connected in line with the borohydride cartridge.
-
(ii)
Follow Steps 1–3 described in ‘18F-FDG reduction to 18F-FDS’.
-
(iii)
Remove 18F-FDG syringe, and replace with a 20 mL syringe containing 20 mL of PBS.
-
(iv)
Elute PBS.
-
(v)
Discard first 10 mL, and keep the remaining 10 mL in the receiving vial.
-
(vi)
Measure pH using pH strips.
-
(vii)
Measure the activity of 18F-FDS eluted and time.
-
(i)
-
(A)
QC of 18F-FDS
Timing 10–20 min
Visual appearance
Caution
Use appropriate radiation shielding (e.g., leaded glass).
-
15
Visually inspect the 18F-FDS solution, and confirm the resulting solution is clear and shows no evidence of foreign matter.
pH test
-
16
Apply drops of 18F-FDS solution to pH indicator paper. Match strip colors to indicator chart. pH is usually 6.
Radiochemical yield
-
17
Transfer the 18F-FDS collection vial to a dose calibrator.
-
18
Record radioactivity and time of reading.
-
19
Calculate RCY using the following equation: RCY (%) = (18F-FDG starting activity)/(18F-FDS collected activity) × 100.
Radiochemical purity and identity
-
20
Prepare 10 × 1.5 cm TLC strips with marked 1 cm baseline from the bottom and 1 cm solvent front from the top.
-
21
Dilute 18F-FDG and 18F-FDS aliquots in Eppendorf tubes with 1 mL water.
Critical
Measure the activity of the aliquots, and dilute further if needed to ensure activity is ~0.5 MBq/µL.
-
22
Using the diluted aliquots from the Eppendorf tubes, spot samples (2 µL) of starting 18F-FDG, eluted 18F-FDS, and a mixture of 18F-FDG + 18F-FDS onto 1 cm baseline of TLC plates.
-
23
Develop the TLC plates in a chamber with 80% acetonitrile in water as the mobile phase.
-
24
Once finished and the plates are dry, scan plates with Eckert + Ziegler AR-2000 radio-TLC imaging scanner (or alternative radio-TLC scanner).
-
25
To confirm chemical identity, Rf from radio-chromatogram must match Rf of the standard FDS (prepared as described in ‘Reagent setup’).
-
26
Calculate the radiochemical purity using the following equation: radiochemical purity = peak area (18F-FDS peak)/sum (all peak areas) × 100. A radiochemical purity >90% is required.
TLC analysis of FDG and FDS
-
27
Prepare 10 × 1.5 cm TLC strips with marked 1 cm baseline from the bottom and 1 cm solvent front from the top.
-
28
Dilute FDG and FDS aliquots in Eppendorf tubes with 1 mL water.
-
29
Spot samples (2 µL) onto 1 cm baseline of TLC plates.
-
30
Develop the TLC plates in a chamber with 80% acetonitrile in water as the mobile phase.
-
31
Once finished and the plates are dry, spray with KMnO4 solution and dry with heating. Spots appear yellow on a purple background.
-
32
Calculate Rf.
Troubleshooting
Troubleshooting advice is provided in Table 3.
Timing
-
Steps 1–2, cartridge pre-equilibration: 3 min
-
Steps 3–6, 18F-FDG preparation: 2 min
-
Steps 7–13, 18F-FDG reduction to 18F-FDS: 5–10 min
-
Step 14, 18F-FDS purification and formulation: 5 min
-
Steps 15–32, QC of 18F-FDS: 10–20 min
Anticipated results
18F-FDS is obtained in >70% RCY as a clear, colorless solution with no visible particulate matter. The solution pH is 6 (between 6 and 8 is acceptable). Radio-TLC shows a spot for 18F-FDS at ~50 mm (Rf 0.6), and unreacted 18F-FDG at 65 mm (Rf 0.7), which matches the TLC Rfs obtained with standard FDG and FDS (within 10%) (Fig. 3).
Radiochemical purity ≥90 % is acceptable. Following these criteria, 18F-FDS can then be used for in vitro or in vivo follow-up experiments (Fig. 4).
a, Setup and timeline of the experiment. Hamsters were intranasally inoculated with SARS-CoV-2 (1.5 × 105 50% tissue culture infective dose) and disease progressed for 7 d, after which a secondary intratracheal infection with K. pneumoniae (ATCC 43816, 3 log10 colony-forming units) was initiated. On day 9, anesthetized animals received 18F-FDS (7.39 ± 1.50 MBq, n = 6) intravenously and PET imaging was performed 120 min post-tracer injection to allow clearance of 18F-FDS from non-infected tissues. PET was acquired for 15 min, followed by CT. b, Three-dimensional maximum intensity projection (MIP), transverse and coronal CT, and overlaid 18F-FDS PET in infected hamsters. Hamsters show 18F-FDS PET signal in the areas of K. pneumoniae pneumonia visible on CT. 18F-FDS signal can also be seen in the kidneys, gallbladder and intestines. Quantification of the PET signal shows that the mean standard uptake values (SUVmean) are significantly higher for areas of pneumonia compared with unaffected lung (P = 0.002). Data represented as median and interquartile range. Statistical analyses were performed using a Mann–Whitney U test. Images adapted and reproduced with permission from Ordonez et al. Sci Transl Med16. Copyright 2021 The American Association for the Advancement of Science.
Following intravenous administration into mammals, 18F-FDS is predominantly cleared through the kidneys and allows for evaluation of renal function. PET/CT imaging performed 2 h post-injection shows minimal uptake in the heart, liver and gastrointestinal tract. 18F-FDS accumulates in regions of infection by Enterobacterales. Repeat imaging following treatment can evaluate treatment success, which is characterized by absent or substantially decreased 18F-FDS signal. Treatment failure can be detected if 18F-FDS signal remains following treatment.
Data availability
All data generated or analyzed during this study are included in this article and the supporting paper16.
References
James, M. L. & Gambhir, S. S. A molecular imaging primer: modalities, imaging agents, and applications. Physiol. Rev. 92, 897–965 (2012).
Jain, S. K. The promise of molecular imaging in the study and treatment of infectious diseases. Mol. Imaging Biol. 19, 341–347 (2017).
Cherry, S. R. et al. Total-body imaging: transforming the role of positron emission tomography. Sci. Transl. Med. 9, eaaf6169 (2017).
International Atomic Energy Agency. IMAGINE—general data overview. https://humanhealth.iaea.org/HHW/DBStatistics/IMAGINEMaps1.html
Wangler, C. et al. One-step 18F-labeling of carbohydrate-conjugated octreotate-derivatives containing a silicon-fluoride-acceptor (SiFA): in vitro and in vivo evaluation as tumor imaging agents for positron emission tomography (PET). Bioconjug. Chem. 21, 2289–2296 (2010).
Basuli, F., Zhang, X., Jagoda, E. M., Choyke, P. L. & Swenson, R. E. Facile room temperature synthesis of fluorine-18 labeled fluoronicotinic acid-2,3,5,6-tetrafluorophenyl ester without azeotropic drying of fluorine-18. Nucl. Med. Biol. 43, 770–772 (2016).
van der Born, D. et al. Fluorine-18 labelled building blocks for PET tracer synthesis. Chem. Soc. Rev. 46, 4709–4773 (2017).
Allott, L., Barnes, C., Brickute, D., Leung, S. F. J. & Aboagye, E. O. Solid-supported cyanoborohydride cartridges for automation of reductive amination radiochemistry. React. Chem. Eng. 4, 1748–1751 (2019).
Liu, Z. et al. One-step 18F labeling of biomolecules using organotrifluoroborates. Nat. Protoc. 10, 1423–1432 (2015).
Wängler, C. et al. One-step 18F-labeling of peptides for positron emission tomography imaging using the SiFA methodology. Nat. Protoc. 7, 1946–1955 (2012).
Donnenberg, M. S. Enterobacteriaceae. in Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases (eds Mandell, G. L. et al.) 2815–2833 (Elsevier, 2010).
Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States, 2019. https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf
O’Neill, J. Tackling drug-resistant infections globally: final report and recommendations (Wellcome Trust and UK Government, 2016).
Tucker, E. W. et al. Noninvasive 11C-rifampin positron emission tomography reveals drug biodistribution in tuberculous meningitis. Sci. Transl. Med. 10, eaau0965 (2018).
Mota, F. et al. Radiotracer development for bacterial imaging. J. Med. Chem. 63, 1964–1977 (2020).
Ordonez, A. A. et al. Imaging Enterobacterales infections in patients using pathogen-specific positron emission tomography. Sci. Transl. Med. 13, eabe9805 (2021).
Gowrishankar, G. et al. Specific imaging of bacterial infection using 6″-(18)F-fluoromaltotriose: a second-generation PET tracer targeting the maltodextrin transporter in bacteria. J. Nucl. Med. 58, 1679–1684 (2017).
Weinstein, E. A. et al. Imaging Enterobacteriaceae infection in vivo with 18F-fluorodeoxysorbitol positron emission tomography. Sci. Transl. Med. 6, 259ra146 (2014).
Mutch, C. A. et al. [11C]Para-aminobenzoic acid: a positron emission tomography tracer targeting bacteria-specific metabolism. ACS Infect. Dis. 4, 1067–1072 (2018).
Ordonez, A. A. et al. A systematic approach for developing bacteria-specific imaging tracers. J. Nucl. Med. 58, 144–150 (2017).
Sellmyer, M. A. et al. Bacterial infection imaging with [(18)F]fluoropropyl-trimethoprim. Proc. Natl Acad. Sci. USA 114, 8372–8377 (2017).
Zhang, Z. et al. Positron emission tomography imaging with 2-[18F]F-p-aminobenzoic acid detects Staphylococcus aureus infections and monitors drug response. ACS Infect. Dis. 4, 1635–1644 (2018).
Petrik, M. et al. Imaging of Pseudomonas aeruginosa infection with Ga-68 labelled pyoverdine for positron emission tomography. Sci. Rep. 8, 15698 (2018).
Parker, M. F. L. et al. Sensing living bacteria in vivo using d-alanine-derived (11)C radiotracers. ACS Cent. Sci. 6, 155–165 (2020).
Vilche, M. et al. (6)(8)Ga-NOTA-UBI-29-41 as a PET tracer for detection of bacterial infection. J. Nucl. Med. 57, 622–627 (2016).
Polvoy, I., Flavell, R. R., Rosenberg, O. S., Ohliger, M. A. & Wilson, D. M. Nuclear imaging of bacterial infection: the state of the art and future directions. J. Nucl. Med. 61, 1708–1716 (2020).
Li, J., Zheng, H., Fodah, R., Warawa, J. M. & Ng, C. K. Validation of 2-(18)F-fluorodeoxysorbitol as a potential radiopharmaceutical for imaging bacterial infection in the lung. J. Nucl. Med. 59, 134–139 (2018).
Yao, S. et al. Infection imaging with (18)F-FDS and first-in-human evaluation. Nucl. Med. Biol. 43, 206–214 (2016).
Ordonez, A. A. et al. Molecular imaging of bacterial infections: overcoming the barriers to clinical translation. Sci. Transl. Med. 11, eaax8251 (2019).
Blaufox, M. D. et al. The SNMMI and EANM practice guideline for renal scintigraphy in adults. Eur. J. Nucl. Med. Mol. Imaging 45, 2218–2228 (2018).
Werner, R. A. et al. The next era of renal radionuclide imaging: novel PET radiotracers. Eur. J. Nucl. Med. Mol. Imaging 46, 1773–1786 (2019).
Toyama, Y. et al. Current and future perspectives on functional molecular imaging in nephro-urology: theranostics on the horizon. Theranostics 11, 6105–6119 (2021).
Zhu, W. et al. Biodistribution and radiation dosimetry of the Enterobacteriaceae-specific imaging probe [(18)F]fluorodeoxysorbitol determined by PET/CT in healthy human volunteers. Mol. Imaging Biol. 18, 782–787 (2016).
Werner, R. A. et al. Functional renal imaging with 2-deoxy-2-(18)F-fluorosorbitol PET in rat models of renal disorders. J. Nucl. Med. 59, 828–832 (2018).
Werner, R. A. et al. Novel functional renal PET imaging with 18F-FDS in human subjects. Clin. Nucl. Med. 44, 410–411 (2019).
Li, Z. B. et al. The synthesis of 18F-FDS and its potential application in molecular imaging. Mol. Imaging Biol. 10, 92–98 (2008).
Hasegawa, K., Koshino, K. & Higuchi, T. Facile synthesis of 2-deoxy-2-[(18) F]fluorosorbitol using sodium borohydride on aluminum oxide. J. Labelled Comp. Radiopharm. 64, 40–46 (2021).
Yao, S. et al. Infection imaging with 18F-FDS and first-in-human evaluation. Nucl. Med. Biol. 43, 206–214 (2016).
Šljukić, B., Santos, D. M., Sequeira, C. A. & Banks, C. E. Analytical monitoring of sodium borohydride. Anal. Methods 5, 829–839 (2013).
Acknowledgements
This work was funded by the U.S. National Institutes of Health (R01-HL131829, R01-AI153349, Director’s Transformative Research Award R01-EB020539), Department of Defense’s Congressionally Directed Medical Research Programs PR-171338P1 and Maryland Innovation Initiative to S.K.J.
Author information
Authors and Affiliations
Contributions
F.M. and S.K.J. conceptualized the project. F.M. and P.D.J. performed the experiments and analyzed the data. All authors contributed to writing the paper.
Corresponding author
Ethics declarations
Competing interests
F.M. and S.K.J. are co-inventors on pending patent USPA #63/071,755 on the solid-phase cartridge to formulate ready-to-use 18F-FDS, filed by Johns Hopkins University. S.K.J. is an inventor on pending patent US20150250906A1 on bacteria-specific labeled substrates as imaging biomarkers, filed by Johns Hopkins University.
Additional information
Peer review information Nature Protocols thanks Zibo Li and Jung-Joon Min for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key reference using this protocol
Ordonez, A. A. et al. Sci. Transl. Med. 13, eabe98052021 (2021): https://doi.org/10.1126/scitranslmed.abe9805
Rights and permissions
About this article
Cite this article
Mota, F., De Jesus, P. & Jain, S.K. Kit-based synthesis of 2-deoxy-2-[18F]-fluoro-d-sorbitol for bacterial imaging. Nat Protoc 16, 5274–5286 (2021). https://doi.org/10.1038/s41596-021-00613-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-021-00613-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.