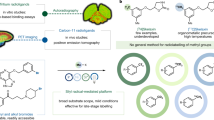
Abstract
Radiotracers labeled with carbon-11 (t1/2 = 20.4 min) are widely used with positron emission tomography for biomedical research. Radiotracers must be produced for positron emission tomography studies in humans according to prescribed time schedules while also meeting current good manufacturing practice. Translation of an experimental radiosynthesis to a current good manufacturing practice environment is challenging. Here we exemplify such translation with a protocol for the production of an emerging radiotracer for imaging brain translocator protein 18 kDa, namely [11C]ER176. This radiotracer is produced by rapid conversion of cyclotron-produced [11C]carbon dioxide into [11C]iodomethane, which is then used to treat N-desmethyl-ER176 in the presence of base (tBuOK) at room temperature for 5 min. [11C]ER176 is separated in high purity by reversed-phase HPLC and formulated for intravenous injection in sterile ethanol–saline. The radiosynthesis is reliable and takes 50 min. Quality control takes another 20 min. All aspects of the protocol, including quality control, are discussed.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Data in this protocol are from the cited literature. Any new data are provided as original source data in the Supplementary Information.
Code availability
Software are deposited at https://github.com/jinsoohong-netizen/ER176
References
Ametamey, S. M., Honer, M. & Schubiger, P. A. Molecular imaging with PET. Chem. Rev. 108, 1501–1516 (2008).
Matthews, P. M., Rabiner, E. A., Passchier, J. & Gunn, R. N. Positron emission tomography molecular imaging for drug development. Br. J. Clin. Pharmacol. 73, 175–186 (2011).
Phelps, M. E. Positron emission tomography provides molecular imaging of biological processes. Proc. Natl Acad. Sci. USA 97, 9226–9233 (2000).
Zanotti-Fregonara, P. et al. Synthesis and evaluation of translocator18 kDa protein (TSPO) positron emission tomography (PET) radioligands with low binding sensitivity to human single nucleotide polymorphism rs6971. ACS Chem. Neurosci. 5, 963–971 (2014).
Fan, J., Lindemann, P., Feuilloley, M. G. & Papadopoulos, V. Structural and functional evolution of the translocator protein (18 kDa). Curr. Mol. Med. 12, 369–386 (2012).
Jaremko, L., Jaremko, M., Giller, K., Becker, S. & Zweckstetter, M. Structure of the mitochondrial translocator protein in complex with a diagnostic ligand. Science 343, 1363–1366 (2014).
Cagnin, A., Gerhard, A. & Banati, R. B. In vivo imaging of neuroinflammation. Eur. Neuropsychopharmacol. 12, 581–586 (2002).
Owen, D. R. J. & Matthews, P. M. Imaging brain microglial activation using positron emission tomography and translocator protein-specific radioligands. Int. Rev. Neurobiol. 101, 19–39 (2011).
Doorduin, J., de Vries, E. F., Dierckx, R. A. & Klein, H. C. PET imaging of the peripheral benzodiazepine receptor: monitoring disease progression and therapy response in neurodegenerative disorders. Curr. Pharmaceut. Design 14, 3297–3315 (2008).
Kreisl, W. C. et al. PET imaging of neuroinflammation in neurologic disorders. Lancet Neurol. 19, 940–950 (2020).
Yasuno, F. et al. Increased binding of peripheral benzodiazepine receptor in Alzheimer’s disease measured by positron emission tomography with [11C]DAA1106. Biol. Psychiatry 64, 835–841 (2008).
Kreisl, W. C. et al. In vivo radioligand binding to translocator protein correlates with severity of Alzheimer’s disease. Brain 136, 2228–2238 (2013).
Kreisl, W. C. et al. 11C-PBR28 binding to translocator protein increases with progression of Alzheimer’s disease. Neurobiol. Aging 44, 53–61 (2016).
Meyer, J. H. et al. Neuroinflammation in psychiatric disorders: PET imaging and promising new targets. Lancet Psychiatry 7, 1064–1074 (2020).
Richards, E. M. et al. PET radioligand binding to translocator protein (TSPO) is increased in unmedicated depressed subjects. EJNMMI Res. 8, 57 (2018).
Camsonne, R. et al. Synthesis of N-(11C) methyl. N-(methyl-1 propyl). (chloro-2 phenyl)-1isoquinoleine carboxamide-3 (PK11195): a new ligand for peripheral benzodiazepine receptors. J. Label. Compd. Radiopharm 21, 985–991 (1984).
Shah, F., Hume, S. P., Pike, V. W., Ashworth, S. & McDermott, J. Synthesis of the enantiomers of [N-methyl-11C]PK 11195 and comparison of their behaviours asradioligands for PK binding sites in rats. Nucl.Med. Biol. 21, 573–581 (1994).
Chauveau, F., Boutin, H., Van, C. N., Dollé, F. & Tavitian, B. Nuclear imaging of neuroinflammation: a comprehensive review of [11C]PK11195 challengers. Eur. J. Nucl. Med. Mol. Imaging 35, 2304–2319 (2008).
Dollè, F., Luus, C., Reynolds, A. J. & Kassiou, M. Radiolabelled molecules for imaging the translocator protein (18 kDa) using positron emission tomography. Curr. Med. Chem. 16, 2899–2923 (2009).
Briard, E. et al. Synthesis and evaluation in monkey of two sensitive 11C-labeled aryloxyanilide ligands for imaging brain peripheral benzodiazepine receptors in vivo. J. Med. Chem. 51, 17–30 (2008).
Fujita, M. et al. Kinetic analysis in healthy humans of a novel positron emission tomography radioligand to image the peripheral benzodiazepine receptor, a potential biomarker for inflammation. Neuroimage 40, 43–52 (2008).
Endres, C. J. et al. Initial evaluation of 11C-DPA-713, a novel TSPO PET ligand, in humans. J. Nucl. Med. 50, 1276–1282 (2009).
Kreisl, W. C. et al. Comparison of [11C]-(R)-PK 11195 and [11C]PBR28, two radioligands for translocator protein (18 kDa) in human and monkey: implications for positron emission tomographic imaging. Neuroimage 49, 2924–2932 (2010).
Fujita, M. et al. Comparison of four 11C-labeled PET ligands to quantify translocator protein 18 kDa (TSPO) in human brain: (R)-PK11195, PBR28, DPA-713, and ER176-based on recent publications that measured specific-to-non-displaceable ratios. EJNMMI Res. 7, 84 (2017).
Kobayashi, M. et al. 11C-DPA-713 has much greater specific binding to translocator protein 18 kDa (TSPO) in human brain than 11C-(R)-PK11195. J. Cereb. Blood Flow Metab. 38, 393–403 (2018).
Owen, D. R. et al. Two binding sites for [3H]PBR28 in human brain: implications for TSPO PET imaging of neuroinflammation. J. Cereb. Blood Flow Metab. 30, 1608–1618 (2010).
Owen, D. R. et al. Mixed-affinity binding in humans with 18-kDa translocator protein ligands. J. Nucl. Med. 52, 24–32 (2011).
Kreisl, W. C. et al. A genetic polymorphism for translocator protein 18 kDa affects both in vitro and in vivo radioligand binding in human brain to this putative biomarker of neuroinflammation. J. Cereb. Blood Flow Metab. 33, 53–58 (2013).
Owen, D. R. et al. An 18-kDa translocator protein (TSPO) polymorphism explains differences in binding affinity of the PET radioligand PBR28. J. Cereb. Blood Flow Metab. 32, 1–5 (2012).
Ikawa, M. et al. 11C-ER176, a radioligand for 18-kDa translocator protein, has adequate sensitivity to robustly image all three affinity genotypes in human brain. J. Nucl. Med. 58, 320–325 (2017).
Zanotti-Fregonara, P. et al. Head-to-head comparison of 11C-PBR28 and 11C-ER176 for quantification of the translocator protein in the human brain. Eur. J. Nucl. Med. Mol. Imaging 46, 1822–1829 (2019).
Marazano, C., Maziere, M., Berger, G. & Comar, D. Synthesis of methyl iodide-11C and formaldehyde-11C. Int. J. Appl. Radiat. Isot. 28, 49–52 (1977).
Långström, B. & Lundqvist, H. The preparation of 11C-methyl iodide and its use in the synthesis of 11C-methyl-L-methionine. Int. J. Appl. Radiat. Isot. 27, 357–363 (1976).
Crouzel, C., Långström, B., Pike, V. W. & Coenen, H. H. Recommendations for a practical production of [11C]methyl iodide. Appl. Radiat. Isot. 38, 601–603 (1987).
Crouzel, C. et al. Radiochemistry automation for PET. in Radiopharmaceuticals for Positron Emission Tomography (eds Stöcklin, G. & Pike, V. W.) 45–89 (Kluwer Academic, 1993).
Hashimoto, K., Inoue, O., Suzuki, K., Yamasaki, T. & Kojima, M. Synthesis and evaluation of 11C-PK 11195 for in vivo study of peripheral-type benzodiazepine receptors using position emission tomography. Ann. Nucl. Med. 3, 63–71 (1989).
Pike, V. W. et al. Radioligands for PET studies of central benzodiazepine receptors and PK (peripheral benzodiazepine) binding sites—current status. Nucl. Med. Biol. 20, 503–525 (1993).
Lee, H. J. et al. A convenient radiolabeling of [11C](R)-PK11195 using loop method in automatic synthesis module. Nucl. Med. Mol. Imaging 43, 337–343 (2009).
Debruyne, J. C. et al. PET visualization of microglia in multiple sclerosis patients using [11C]PK11195. Eur. J. Neurol. 10, 257–264 (2003).
Coenen, H. H. et al. Consensus nomenclature rules for radiopharmaceutical chemistry—setting the record straight. Nucl. Med. Biol. 55, v–xi (2017).
Link, J. M., Krohn, K. A. & Clark, J. C. Production of [11C]CH3I by single pass reaction of [11C]CH4 with I2. Nucl. Med. Biol. 24, 93–97 (1997).
Larsen, P., Ulin, J., Dahlstrøm, K. & Jensen, M. Synthesis of [11C]iodomethane by iodination of [11C]methane. Appl. Radiat. Isot. 48, 153–157 (1997).
Christman, D. R., Finn, R. D., Karlstrom, K. I. & Wolf, A. P. The production of ultra high activity 11C-labelled hydrogen cyanide, carbon dioxide, carbon monoxide, and methane via the 14N(p,α)11C reaction. Int. J. Appl. Radiat. Isot. 26, 435–442 (1975).
Wilson, A. A., Garcia, A., Jin, L. & Houle, S. Radiotracer synthesis from [11C]-iodomethane: a remarkably simple captive solvent method. Nucl. Med. Biol. 27, 529–532 (2000).
Jewett, D. M., Magner, T. J. & Watkins, G. L. Captive solvent methods for fast, simple carbon-11 radio-alkylations. in New Trends in Radiopharmaceutical Synthesis, Quality Assurance, and Regulatory Control (ed. Emran, A. M.) 387–391 (Plenum Press, 1991).
Shao, X., Schnau, P. L., Fawaz, M. & Scott, P. J. H. Enhanced radiosyntheses of [11C]raclopride and [11C]DASB using ethanolic loop chemistry. Nucl. Med. Biol. 40, 109–116 (2013).
Dahl, K. et al. “In-loop” [11C]CO2 fixation: prototype and proof of concept. J. Label. Compd. Radiopharm 61, 252–262 (2018).
Ferrat, M., Halldin, C., Dahl, K. & Schou, M. “In loop” carbonylation—a simplified method for carbon-11 labeling of drugs and radioligands. J. Label. Compd. Radiopharm 63, 100–107 (2020).
Rahman, O., Kihlberg, T. & Långström, B. Synthesis of N-methyl-N-(1-methylpropyl)-1-(2-chlorophenyl)- isoquinoline-3-[11C]carboxamide ([11C-carbonyl]PK11195) and some analogues using [11C]carbon monoxide and 1-(2-chlorophenyl)isoquinolin-3-yl triflate. J. Chem. Soc., Perkin Trans. 1, 2699–2703 (2002).
Pike, V. W. PET Radiotracers: crossing the blood-brain barrier and surviving metabolism. Trends Pharmacol. Sci. 30, 431–440 (2009).
Castellano, S. et al. Synthesis and biological evaluation of 4-phenylquinazoline-2-carboxamides designed as a novel class of potent ligands of the translocator protein. J. Med. Chem. 55, 4506–4510 (2012).
General Chapter <823> Radiopharmaceuticals For Positron Emission Tomography—Compounding. in The United States Pharmacopeia, 24th rev., and The National Formulary, 19th ed. Rockville, MD: United States Pharmacopeial Convention, 1988–1990 (2000).
US Food and Drug Administration, Department of Health and Human Services; Current good manufacturing practice for positron emission tomography drugs. Code of Federal Regulations, Title 21, vol. 4, Part 212 (revised April 1, 2020).
Qaim, S. M. et al.. PET radionuclide production in PET. in Radiopharmaceuticals for Positron Emission Tomography (eds Stöcklin, G. & Pike, V. W.) 1–44 (Kluwer Academic, 1993).
Bida, G., Ruth, T. J. & Wolf, A. P. Experimentally determined thick target yields for the 14N(p, α)11C reaction. Radiochim. Acta 27, 181–185 (1980).
Buckley, K. R., Huser, J., Jivan, S., Chun, K. S. & Ruth, T. J. 11C-Methane production in small volume, high pressure gas targets. Radiochim. Acta 88, 201–205 (2000).
Suzuki, K., Yamazaki, I., Sasaki, M. & Kubodera, A. Specific activity of [11C]CO2 generated in a N2 gas target: effect of irradiation dose, irradiation history, oxygen content and beam energy. Radiochim. Acta 88, 211–216 (2000).
Gómez-Vallejo, V., Gaja, V., Koziorowski, J. & Llop, J. Specific activity of 11C-labelled radiotracers: a big challenge for PET chemists. in Positron Emission Tomography—Current Clinical and Research Aspects (ed. Hsieh, C.-H.) 183–210 (Intechopen.com, 2012).
wa Kitwanga, S., Leleux, P., Lipnik, P. & Vanhorenbeeck, J. Production of 14,15O, 18F, and 19Ne radioactive nuclei from (p, n) reactions up to 30 MeV. Phys. Rev. C 42, 748–752 (1990).
Jewett, D. M. A simple synthesis of [11C]methyl triflate. Appl. Radiat. Isot. 43, 1383–1385 (1992).
Mathis, C. A. et al. Synthesis and evaluation of 11C-labeled 6-substituted 2-arylbenzothiazoles as amyloid imaging agents. J. Med. Chem. 46, 2740–2754 (2003).
Solbach, C., Uebele, M., Reischl, G. & Machulla, H.-J. Efficient radiosynthesis of carbon-11 labelled uncharged Thioflavin T derivatives using [11C]methyl triflate for β-amyloid imaging in Alzheimer’s Disease with PET. Appl. Rad. Isot. 62, 591–595 (2005).
Matarrese, M. et al. Labeling and evaluation of N-[11C]methylated quinoline-2-carboxamides as potential radioligands for visualization of peripheral benzodiazepine receptors. J. Med. Chem. 44, 579–585 (2001).
Cleij, M. C., Aigbirhio, F. I., Baron, J.-C. & Clark, J. C. Base-promoted dechlorination of (R)-[11C]PK11195. J. Label. Compd. Radiopharm 46, S88 (2003).
Ikoma, T. et al. Radicals in the mechanochemical dechlorination of hazardous organochlorine compounds using CaO nanoparticles radicals in mechanochemical dechlorination. Bull. Chem. Soc. Jpn. 74, 2303–2309 (2001).
Tanaka, Y., Zhang, Q. & Saito, F. Mechanochemical dechlorination of trichlorobenzene on oxide surfaces. J. Phys. Chem. B 107, 11091–11097 (2003).
Tanaka, Y., Zhang, Q., Saito, F., Ikoma, T. & Tero-Kubota, S. Dependence of mechanochemically induced decomposition of mono-chlorobiphenyl on the occurrence of radicals. Chemosphere 60, 939–943 (2005).
Lu, S., Huang, J., Peng, Z., Li, X. & Yan, J. Ball milling 2,4,6-trichlorophenol with calcium oxide: dechlorination experiment and mechanism considerations. Chem. Eng. J. 195–196, 62–68 (2012).
Gómez-Vallejo, V. & Llop, J. Fully automated and reproducible radiosynthesis of high specific activity [¹¹C]raclopride and [¹¹C]Pittsburgh compound-B using the combination of two commercial synthesizers. Nucl. Med. Commun. 32, 1011–1017 (2011).
Verdurand, M. et al. Automated radiosynthesis of the Pittsburg compound-B using a commercial synthesizer. Nucl. Med. Commun. 29, 920–926 (2008).
Canales-Candela, R., Riss, P. J. & Aigbirhio, F. I. Synthesis of [11C]flumazenil ([11C]FMZ). in Radiochemical Syntheses, Vol. 1: Radiopharmaceuticals for Positron Emission Tomography (eds Hockley, B. G. & Scott, P. J. H.) 221‒231 (John Wiley and Sons, 2012).
Shao, X. & Kilbourn, M. R. A simple modification of GE tracerlab FX C Pro for rapid sequential preparation of [11C]carfentanil and [11C]raclopride. Appl. Radiat. Isot. 67, 602–605 (2009).
Shao, X. et al. Highlighting the versatility of the Tracerlab synthesis modules. Part 2: fully automated production of [11C]-labeled radiopharmaceuticals using a Tracerlab FXC-Pro. J. Label. Compd. Radiopharm. 54, 819–838 (2011).
Houle, S. & Vasdev, N. Utility of commercial radiosynthetic modules in captive solvent [11C]-methylation reactions. J. Label. Compd. Radiopharm. 52, 490–492 (2009).
Collier, T. L. et al. Synthesis and preliminary PET imaging of 11C and 18F isotopologues of the ROS1/ALK inhibitor lorlatinib. Nat. Commun. 8, 15761 (2017).
James, M. L. et al. Synthesis and in vivo evaluation of a novel peripheral benzodiazepine receptor PET radioligand. Bioorg. Med. Chem. 13, 6188–6194 (2005).
Cremer, J. E. et al. The distribution of radioactivity in brains of ratsgiven [N-methyl-11C]PK 11195 in vivo after induction of a corticalischaemic lesion. Nucl.Med. Biol. 19, 159–166 (1992).
Acknowledgements
This work was supported by the Intramural Research Program of the National Institutes of Health (National Institute of Mental Health project number ZIA-MH002793). The authors are grateful to the NIH Clinical Center PET Department (Chief: P. Herscovitch) for cyclotron production of carbon-11. The authors thank S. Lu (NIMH) for careful checking of the manuscript.
Author information
Authors and Affiliations
Contributions
V.W.P. conceived and supervised the project. Y.Z, S.T. and C.L.M. developed the radiochemistry. J.H. developed the automated radiosynthesis procedure and analytical methods. S.T., C.L.M., W.H.M. and J.H. performed productions and analyses. H.U.S. performed mass spectrometry. All authors analyzed the data, and read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Protocols thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key reference using this protocol
Fujita, M. et al. EJNMMI Res. 7, 84 (2017): https://ejnmmires.springeropen.com/track/pdf/10.1186/s13550-017-0334-8.pdf
Extended data
Extended Data Fig. 1
Overall setup of the [11C]ER176 production apparatus.
Supplementary information
Supplementary Information
Supplementary Methods 1–9, Supplementary Figs. 1–8 and Supplementary Tables 1–6.
Rights and permissions
About this article
Cite this article
Hong, J., Telu, S., Zhang, Y. et al. Translation of 11C-labeled tracer synthesis to a CGMP environment as exemplified by [11C]ER176 for PET imaging of human TSPO. Nat Protoc 16, 4419–4445 (2021). https://doi.org/10.1038/s41596-021-00584-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-021-00584-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.