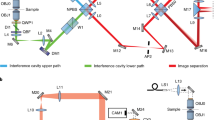
Abstract
Fluorescence microscopy has become an indispensable tool for cell biology. Recently, super-resolution methods have been developed to overcome the diffraction limit of light and have shown living cells in unprecedented detail. Often, these methods come at a high cost and with complexity in terms of instrumentation and sample preparation, thus calling for the development of low-cost, more accessible methods. We previously developed image scanning microscopy (ISM), which uses structured illumination to double the resolution and quadruple the contrast of a confocal microscope. Implementing this technique into a confocal spinning-disk (CSD) microscope allows recording ISM images with up to ~1 frame per second, making it ideal for imaging dynamic biological processes. Here we present a step-by-step protocol describing how to convert any existing commercial CSD microscope into a CSD-ISM, with only moderate changes to the hardware and at low cost. Operation of the CSD-ISM is realized with a field programmable gate array using the software environment Micro-Manager, a popular open-source platform for microscopy. The provided software (https://projects.gwdg.de/projects/csdism-2020) takes care of all algorithmic complexities and numerical workload of the CSD-ISM, including hardware synchronization and image reconstruction. The hardware modifications described here result in a theoretical maximum increase in resolution of √2 ≈ 1.41, which can be further improved by deconvolution to obtain a theoretical maximum twofold increase. An existing CSD setup can be upgraded in ~3 d by anyone with basic knowledge in optics, electronics and microscopy software.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data presented in Fig. 3 (calibration images), Fig. 4 (ISM example images of beads and cells) and Supplementary Fig. 7 were generated for this protocol. The raw data files for Fig. 3 can be found at https://doi.org/10.25625/WTE3OI, and the raw data files for Fig. 4 can be found at https://doi.org/10.25625/QVWCNT.
Code availability
The presented software for CSD-ISM acquisition and reconstruction, including demo data, is available at https://projects.gwdg.de/projects/csdism-2020.
References
Hell, S. W. & Wichmann, J. Breaking the diffraction resolution limit by stimulated emission: stimulated-emission-depletion fluorescence microscopy. Opt. Lett. 19, 780–782 (1994).
Rust, M. J., Bates, M. & Zhuang, X. Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM). Nat. Methods 3, 793–796 (2006).
Betzig, E. et al. Imaging intracellular fluorescent proteins at nanometer resolution. Science 313, 1642–1645 (2006).
Gustafsson, M. G. L. Surpassing the lateral resolution limit by a factor of two using structured illumination microscopy. J. Microsc. 198, 82–87 (2000).
Sheppard, C. J. Super-resolution in confocal imaging. Optik 80, 53–54 (1988).
Müller, C. B. & Enderlein, J. Image scanning microscopy. Phys. Rev. Lett. 104, 198101 (2010).
Schulz, O. et al. Resolution doubling in fluorescence microscopy with confocal spinning-disk image scanning microscopy. Proc. Natl Acad. Sci. USA 110, 21000–21005 (2013).
Stuurman, N., Amdodaj, N. & Vale, R. μManager: open source software for light microscope imaging. Microsc. Today 15, 42–43 (2007).
Edelstein, A. D., Amodaj, N., Hoover, K., Vale, R. & Stuurman, N. Computer control of microscopes using µManager. Curr. Protoc. Mol. Biol. 92, 14.20.1–14.20.17 (2010).
Edelstein, A. D. et al. Advanced methods of microscope control using µManager software. J. Biol. Methods 1, e10 (2014).
Dertinger, T., Colyer, R., Iyer, G., Weiss, S. & Enderlein, J. Fast, background-free, 3D super-resolution optical fluctuation imaging (SOFI). Proc. Natl Acad. Sci. USA 106, 22287–22292 (2009).
Hayashi, S. Resolution doubling using confocal microscopy via analogy with structured illumination microscopy. Jpn. J. Appl. Phys. 55, 082501 (2016).
Sheppard, C. J., Mehta, S. B. & Heintzmann, R. Superresolution by image scanning microscopy using pixel reassignment. Opt. Lett. 38, 2889–2892 (2013).
Sheppard, C. J., Castello, M., Tortarolo, G., Vicidomini, G. & Diaspro, A. Image formation in image scanning microscopy, including the case of two-photon excitation. JOSA A 34, 1339–1350 (2017).
Van den Eynde, R. et al. Quantitative comparison of camera technologies for cost-effective super-resolution optical fluctuation imaging (SOFI). J. Phys. Photonics 1, 044001 (2019).
Toomre, D. & Pawley, J. B.. in Handbook of Biological Confocal Microscopy 3rd ed (ed Pawley, J.) 221–237 (Springer, 2006).
Nakano, A. Spinning-disk confocal microscopy—a cutting-edge tool for imaging of membrane traffic. Cell Struct. Funct. 27, 349–355 (2002).
Acknowledgements
We thank E. Butkevich for preparation of the cell samples and A. Chizhik for the design of Fig. 1. We thank M. Schönekeß from our institute’s electronics workshop for providing Supplementary Fig. 4. S.Q. acknowledges funding from the European Research Council via its Horizon 2020 Framework Programme (675512, BE-OPTICAL). S.I. acknowledges financial support from the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) via project A11 of SFB 937. J.E. acknowledges funding from DFG under Germany’s Excellence Strategy - EXC 2067/1-390729940.
Author information
Authors and Affiliations
Contributions
J.E. conceived the project and designed the experiments. S.Q. developed the software. S.I. and I.G. performed experiments. S.Q. and S.I. analyzed the data. S.Q. and S.I. wrote the manuscript with the input of all other authors, J.E. revised and performed final edits to the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Protocols thanks Lucien Weiss and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Schulz, O. et al. Proc. Natl Aad. Sci. USA 110, 21000–21005 (2013): https://doi.org/10.1073/pnas.1315858110
Müller, C. B. & Enderlein, J. Phys. Rev. Lett. 104, 198101 (2010): https://doi.org/10.1103/PhysRevLett.104.198101
Gregor, I. et al. Nat. Methods 14, 1087–1089 (2017): https://doi.org/10.1038/nmeth.4467
Supplementary information
Supplementary Information
Supplementary Note 1, Supplementary Figs. 1–7 and Supplementary Table 1.
Supplementary Video 1
Screen-captured video explaining the use of the Micro-Manager plugin.
Supplementary Video 2
Screen-captured video explaining the use of the reconstruction software.
Supplementary Video 3
This video illustrates the data acquisition and the ISM reconstruction of the bead image in Fig. 4a,b.
Supplementary Video 4
This video illustrates the data acquisition and the ISM reconstruction of the cell image in Fig. 4g,h.
Rights and permissions
About this article
Cite this article
Qin, S., Isbaner, S., Gregor, I. et al. Doubling the resolution of a confocal spinning-disk microscope using image scanning microscopy. Nat Protoc 16, 164–181 (2021). https://doi.org/10.1038/s41596-020-00408-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-020-00408-x
This article is cited by
-
Three-dimensional operando optical imaging of particle and electrolyte heterogeneities inside Li-ion batteries
Nature Nanotechnology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.