Abstract
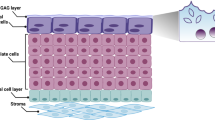
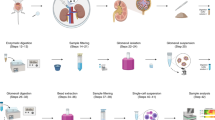
Urothelial cells contribute to bladder functions, including urine storage, urine emptying, and innate immune response. Functional studies of urothelial cells usually use either freshly isolated cells or cultured cells. Most methods of isolating urothelial cells require enzymes; however, these techniques remove proteins that connect the cells and disrupt the orientation of the cells within the multilayered urothelium. In addition, PCR or immunoblot results obtained from homogenates of bladder mucosa or whole bladder do not represent pure urothelial cells. We describe a dissection process that does not require enzymes and is able to obtain pure urothelial tissues from mice and humans. This method can isolate single urothelial cells for electrophysiology in situ and can also isolate pure urothelial tissue for PCR, microarray, and immunoblot procedures. The time required to obtain urothelial tissue from one mouse bladder is 15–20 min. This method is simple and time efficient as compared with alternative methods and therefore facilitates our understanding of urothelial biology.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Birder, L. & Andersson, K. E. Urothelial signaling. Physiol. Rev. 93, 653–680 (2013).
Hamilton, C., Tan, L., Miethke, T. & Anand, P. K. Immunity to uropathogens: the emerging roles of inflammasomes. Nat. Rev. Urol. 14, 284–295 (2017).
Chai, T. C., Russo, A., Yu, S. & Lu, M. Mucosal signaling in the bladder. Auton. Neurosci. 200, 49–56 (2016).
Balsara, Z. R. & Li, X. Sleeping beauty: awakening urothelium from its slumber. Am. J. Physiol. Renal Physiol. 312, F732–F743 (2017).
Watanabe, M. et al. Trpm7 protein contributes to intercellular junction formation in mouse urothelium. J. Biol. Chem. 290, 29882–29892 (2015).
Miyamoto, T. et al. Functional role for Piezo1 in stretch-evoked Ca2+ influx and ATP release in urothelial cell cultures. J. Biol. Chem. 289, 16565–16575 (2014).
Mochizuki, T. et al. The TRPV4 cation channel mediates stretch-evoked Ca2+ influx and ATP release in primary urothelial cell cultures. J. Biol. Chem. 284, 21257–21264 (2009).
Everaerts, W. et al. Inhibition of the cation channel TRPV4 improves bladder function in mice and rats with cyclophosphamide-induced cystitis. Proc. Natl. Acad. Sci. USA 107, 19084–19089 (2010).
Kullmann, F. A. et al. Inflammation and tissue remodeling in the bladder and urethra in feline interstitial cystitis. Front. Syst. Neurosci. 12, 13 (2018).
Beckel, J. M. et al. Pannexin 1 channels mediate the release of ATP into the lumen of the rat urinary bladder. J. Physiol. 593, 1857–1871 (2015).
Yang, W., Searl, T. J., Yaggie, R., Schaeffer, A. J. & Klumpp, D. J. A MAPP network study: overexpression of tumor necrosis factor-α in mouse urothelium mimics interstitial cystitis. Am. J. Physiol. Renal Physiol. 315, F36–F44 (2018).
Li, Y., Lu, M., Alvarez-Lugo, L., Chen, G. & Chai, T. C. Granulocyte-macrophage colony-stimulating factor (GM-CSF) is released by female mouse bladder urothelial cells and expressed by the urothelium as an early response to lipopolysaccharides (LPS). Neurourol. Urodyn. 36, 1020–1025 (2017).
Lu, M. et al. Lipopolysaccharide stimulates BK channel activity in bladder umbrella cells. Am. J. Physiol. Cell Physiol. 314, C643–C653 (2018).
Acevedo-Alvarez, M. et al. Mouse urothelial genes associated with voiding behavior changes after ovariectomy and bladder lipopolysaccharide exposure. Neurourol. Urodyn. 37, 2398–2405 (2018).
Niemczyk, G., Czarzasta, K., Radziszewski, P., Wlodarski, P. & Cudnoch-Jedrzejewska, A. Pathophysiological effect of bladder outlet obstruction on the urothelium. Ultrastruct. Pathol. 42, 317–322 (2018).
Park, E. C. et al. Proteomic analysis of urothelium of rats with detrusor overactivity induced by bladder outlet obstruction. Mol. Cell. Proteomics 17, 948–960 (2018).
Yang, S. W., Jeong, S. W. & Song, K. H. Increased expression of neuregulin 1 in the urothelium of rat bladder with partial bladder outlet obstruction. BMC Urol. 17, 115 (2017).
Stenqvist, J., Carlsson, T., Winder, M. & Aronsson, P. Effects of caveolae depletion and urothelial denudation on purinergic and cholinergic signaling in healthy and cyclophosphamide-induced cystitis in the rat bladder. Auton. Neurosci. 213, 60–70 (2018).
Zhang, X. et al. Carbenoxolone inhibits TRPV4 channel-initiated oxidative urothelial injury and ameliorates cyclophosphamide-induced bladder dysfunction. J. Cell. Mol. Med. 21, 1791–1802 (2017).
Lopez-Beltran, A., Montironi, R., Raspollini, M. R., Cheng, L. & Netto, G. J. Iatrogenic pathology of the urinary bladder. Semin. Diagn. Pathol. 35, 218–227 (2018).
Doyle, C. et al. The role of the mucosa in modulation of evoked responses in the spinal cord injured rat bladder. Neurourol. Urodyn. 37, 1583–1593 (2018).
Kullmann, F. A. et al. Urothelial proliferation and regeneration after spinal cord injury. Am. J. Physiol. Renal Physiol. 313, F85–F102 (2017).
Ungerer, T. D. et al. Influence of urothelial or suburothelial cholinergic receptors on bladder reflexes in chronic spinal cord injured cats. Exp. Neurol. 285, 147–158 (2016).
Ozbilgin, M. K. et al. Effects of cyclooxygenase on the urothelium of the urinary bladder of mice exposed to pelvic radiation. Anal. Quant. Cytopathol. Histopathol. 38, 103–110 (2016).
Kanai, A. J. et al. Manganese superoxide dismutase gene therapy protects against irradiation-induced cystitis. Am. J. Physiol. Renal Physiol. 283, F1304–F1312 (2002).
Chang, A. et al. Social stress in mice induces voiding dysfunction and bladder wall remodeling. Am. J. Physiol. Renal Physiol. 297, F1101–F1108 (2009).
Hawthorn, M. H., Chapple, C. R., Cock, M. & Chess-Williams, R. Urothelium-derived inhibitory factor(s) influences on detrusor muscle contractility in vitro. Br. J. Pharmacol. 129, 416–419 (2000).
Andersson, M., Aronsson, P., Doufish, D., Lampert, A. & Tobin, G. Muscarinic receptor subtypes involved in urothelium-derived relaxatory effects in the inflamed rat urinary bladder. Auton. Neurosci. 170, 5–11 (2012).
Chaiyaprasithi, B., Mang, C. F., Kilbinger, H. & Hohenfellner, M. Inhibition of human detrusor contraction by a urothelium derived factor. J. Urol. 170, 1897–1900 (2003).
Saban, M. R., Nguyen, N. B., Hammond, T. G. & Saban, R. Gene expression profiling of mouse bladder inflammatory responses to LPS, substance P, and antigen-stimulation. Am. J. Pathol. 160, 2095–2110 (2002).
Li, M., Sun, Y., Simard, J. M., Wang, J. Y. & Chai, T. C. Augmented bladder urothelial polyamine signaling and block of BK channel in the pathophysiology of overactive bladder syndrome. Am. J. Physiol. Cell Physiol. 297, C1445–C1451 (2009).
National Research Council Committee for the Update of the Guide for the Care and Use of Laboratory Animals. in Guide for the Care and Use of Laboratory Animals (National Academies Press, Washington, DC, 2011).
Deng, F. M. et al. Uroplakin IIIb, a urothelial differentiation marker, dimerizes with uroplakin Ib as an early step of urothelial plaque assembly. J. Cell Biol. 159, 685–694 (2002).
Acknowledgements
We thank L. Sanders for editing the manuscript, L. Alvarez-Lugo for RT–PCR assistance, J. Lu for narration of the video, and K. Dong for help in taking H&E images. This paper was supported by Yale University institutional funding.
Author information
Authors and Affiliations
Contributions
M.L. and T.C.C. conceived and designed the research. M.L. demonstrated the dissection and edited the video. M.L. and K.Z. performed the experiments. M.L. and T.C.C. drafted the manuscript. M.L., P.G.S., and T.C.C. edited and revised the manuscript. All the authors approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Journal peer review information: Nature Protocols thanks David Klumpp and Hiroshi Miyamoto for their contribution to the peer review of this work.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key reference(s) using this protocol
Li, Y., Lu, M., Alvarez-Lugo, L., Chen, G. & Chai, T. C. Neurourol. Urodyn. 36, 1020–1025 (2017): https://doi.org/10.1002/nau.23057
Lu, M. et al. Am. J. Physiol. Cell Physiol. 314, C643–C653 (2018): https://doi.org/10.1152/ajpcell.00339.2017
Acevedo-Alvarez, M. et al. Neurourol. Urodyn. 37, 2398–2405 (2018): https://doi.org/10.1002/nau.23592
Integrated supplementary information
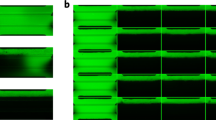
Supplementary Figure 1 Dissected sheet of human urothelial tissue under a microscope.
A, B and C are the same dissected sheet of urothelial tissue from a human bladder at different magnifications. The basal cells are shown facing up. The underlining intermediate cells and umbrella cells could not be seen without removal of the basal cells. Scale bars in: A = 400 µm; B = 200 µm; C = 100 µm. (Human bladder obtained from cystectomy specimens. The Yale IRB approved the protocol.).
Supplementary information
Supplementary Text and Figures
Supplementary Figure 1
Supplementary Video 1
How to dissect urothelial tissue from mouse bladder. Part 1: making the dissection ice dish. Supplementary Video 1 © The Author(s), under license to Springer Nature America, Inc. 2019.
Supplementary Video 2
How to dissect urothelial tissue from mouse bladder. Part 2: perfusing the mouse with PBS. Supplementary Video 2 © The Author(s), under license to Springer Nature America, Inc. 2019.
Supplementary Video 3
How to dissect urothelial tissue from mouse bladder. Part 3: dissecting urothelial tissue. Supplementary Video 3 © The Author(s), under license to Springer Nature America, Inc. 2019.
Rights and permissions
About this article
Cite this article
Lu, M., Zhu, K., Schulam, P.G. et al. A non-enzymatic method for dissection of mouse bladder urothelial tissue. Nat Protoc 14, 1280–1292 (2019). https://doi.org/10.1038/s41596-019-0142-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-019-0142-x
This article is cited by
-
Tailor-made natural and synthetic grafts for precise urethral reconstruction
Journal of Nanobiotechnology (2022)
-
Single-cell analysis reveals urothelial cell heterogeneity and regenerative cues following cyclophosphamide-induced bladder injury
Cell Death & Disease (2021)
-
Innervation: the missing link for biofabricated tissues and organs
npj Regenerative Medicine (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.