Abstract
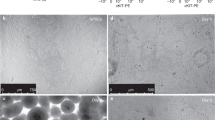
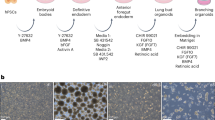
The lung epithelium is derived from the endodermal germ layer, which undergoes a complex series of endoderm–mesoderm-mediated signaling events to generate the final arborized network of conducting airways (bronchi, bronchioles) and gas-exchanging units (alveoli). These stages include endoderm induction, anterior–posterior and dorsal–ventral patterning, lung specification, lung budding, branching morphogenesis, and, finally, maturation. Here we describe a protocol that recapitulates several of these milestones in order to differentiate human pluripotent stem cells (hPSCs) into ventral–anterior foregut spheroids and further into two distinct types of organoids: human lung organoids and bud tip progenitor organoids. The resulting human lung organoids possess cell types and structures that resemble the bronchi/bronchioles of the developing human airway surrounded by lung mesenchyme and cells expressing alveolar-cell markers. The bud tip progenitor organoids possess a population of highly proliferative multipotent cells with in vitro multilineage differentiation potential and in vivo engraftment potential. Human lung organoids can be generated from hPSCs in 50–85 d, and bud tip progenitor organoids can be generated in 22 d. The two hPSC-derived models presented here have been benchmarked with human fetal tissue and found to be representative of human fetal-like tissue. The bud tip progenitor organoids are thus ideal for exploring epithelial fate decisions, while the human lung organoids can be used to model epithelial–mesenchymal cross-talk during human lung development. In addition to their applications in developmental biology, human lung organoids and bud tip progenitor organoids may be implemented in regenerative medicine, tissue engineering, and pharmaceutical safety and efficacy testing.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
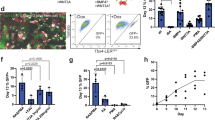
Some of the data presented in the current study were generated for and published in previous reports. Original data used for figures in this paper are available at the following links: Fig. 2b–d (Dye et al.16), https://doi.org/10.7554/eLife.19732; Figs. 2e and 5g–j (Dye et al.15), https://doi.org/10.7554/eLife.05098; Figs. 2g–i and 5l–r (Miller et al.17), https://doi.org/10.1016/j.stemcr.2017.11.012.
References
Zorn, A. M. & Wells, J. M. Vertebrate endoderm development and organ formation. Annu. Rev. Cell Dev. Biol. 25, 221–251 (2009).
Chang, D. R. et al. Lung epithelial branching program antagonizes alveolar differentiation. Proc. Natl. Acad. Sci. USA 110, 18042–18051 (2013).
Rawlins, E. L. Lung epithelial progenitor cells: lessons from development. Proc. Am. Thorac. Soc. 5, 675–681 (2008).
Rawlins, E. L., Clark, C. P., Xue, Y. & Hogan, B. L. M. The Id2+ distal tip lung epithelium contains individual multipotent embryonic progenitor cells. Development 136, 3741–3745 (2009).
Spence, J. R. et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 470, 105–109 (2011).
McCracken, K. W., Howell, J. C., Wells, J. M. & Spence, J. R. Generating human intestinal tissue from pluripotent stem cells in vitro. Nat. Protoc. 6, 1920–1928 (2011).
Múnera, J. O. et al. Differentiation of human pluripotent stem cells into colonic organoids via transient activation of BMP signaling. Cell Stem Cell 21, 51–64 (2017).
Tsai, Y.-H. et al. In vitro patterning of pluripotent stem cell-derived intestine recapitulates in vivo human development. Development 144, 1045–1055 (2017).
Hannan, N. R. F., Sampaziotis, F., Segeritz, C.-P., Hanley, N. A. & Vallier, L. Generation of distal airway epithelium from multipotent human foregut stem cells. Stem Cells Dev. 24, 1680–1690 (2015).
Hannan, N. R. F. et al. Generation of multipotent foregut stem cells from human pluripotent stem cells. Stem Cell Rep. 1, 293–306 (2013).
Longmire, T. A. et al. Efficient derivation of purified lung and thyroid progenitors from embryonic stem cells. Stem Cell 10, 398–411 (2012).
Huang, S. X. L. et al. Efficient generation of lung and airway epithelial cells from human pluripotent stem cells. Nat. Biotechnol. 32, 84–91 (2013).
Green, M. D. et al. Generation of anterior foregut endoderm from human embryonic and induced pluripotent stem cells. Nat. Biotechnol. 29, 267–272 (2011).
Huang, S. X. L. et al. The in vitro generation of lung and airway progenitor cells from human pluripotent stem cells. Nat. Protoc. 10, 413–425 (2015).
Dye, B. R. et al. In vitro generation of human pluripotent stem cell derived lung organoids. Elife 4, e05098 (2015).
Dye, B. R. et al. A bioengineered niche promotes in vivo engraftment and maturation of pluripotent stem cell derived human lung organoids. Elife 5, e19732 (2016).
Miller, A. J. et al. In vitro induction and in vivo engraftment of lung bud tip progenitor cells derived from human pluripotent stem cells. Stem Cell Rep. 10, 101–119 (2018).
Cruz-Acuña, R. et al. Synthetic hydrogels for human intestinal organoid generation and colonic wound repair. Nat. Cell Biol. 19, 1326–1335 (2017).
Szenker-Ravi, E. et al. RSPO2 inhibition of RNF43 and ZNRF3 governs limb development independently of LGR4/5/6. Nature 557, 564–569 (2018).
Hill, D. R. et al. Bacterial colonization stimulates a complex physiological response in the immature human intestinal epithelium. Elife 6, e29132 (2017).
Hill, D. R., Huang, S., Tsai, Y.-H., Spence, J. R. & Young, V. B. Real-time measurement of epithelial barrier permeability in human intestinal organoids. J. Vis. Exp. https://doi.org/10.3791/56960 (2017).
Perrin, S. Preclinical research: make mouse studies work. Nature 507, 423–425 (2014).
Ruggeri, B. A., Camp, F. & Miknyoczki, S. Animal models of disease: pre-clinical animal models of cancer and their applications and utility in drug discovery. Biochem. Pharmacol. 87, 150–161 (2014).
van der Laan, J. W., Chapin, R. E., Haenen, B., Jacobs, A. C. & Piersma, A. Testing strategies for embryo-fetal toxicity of human pharmaceuticals. Animal models vs. in vitro approaches: a workshop report. Regul. Toxicol. Pharmacol. 63, 115–123 (2012).
D’Amour, K. A. et al. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat. Biotechnol. 23, 1534–1541 (2005).
Jacob, A. et al. Differentiation of human pluripotent stem cells into functional lung alveolar epithelial cells. Cell Stem Cell 21, 472–488 (2017).
McCauley, K. B. et al. Efficient derivation of functional human airway epithelium from pluripotent stem cells via temporal regulation of Wnt signaling. Cell Stem Cell 20, 844–857 (2017).
Chen, Y.-W. et al. A three-dimensional model of human lung development and disease from pluripotent stem cells. Nat. Cell Biol. 19, 542–549 (2017).
Gotoh, S. et al. Generation of alveolar epithelial spheroids via isolated progenitor cells from human pluripotent stem cells. Stem Cell Rep. 3, 394–403 (2014).
Konishi, S. et al. Directed induction of functional multi-ciliated cells in proximal airway epithelial spheroids from human pluripotent stem cells. Stem Cell Rep. 6, 18–25 (2016).
Hawkins, F. et al. Prospective isolation of NKX2-1-expressing human lung progenitors derived from pluripotent stem cells. J. Clin. Invest. 127, 2277–2294 (2017).
Wang, D., Haviland, D. L., Burns, A. R., Zsigmond, E. & Wetsel, R. A. A pure population of lung alveolar epithelial type II cells derived from human embryonic stem cells. Proc. Natl. Acad. Sci. USA 104, 4449–4454 (2007).
Firth, A. L. et al. Generation of multiciliated cells in functional airway epithelia from human induced pluripotent stem cells. Proc. Natl. Acad. Sci. USA 111, E1723–E1730 (2014).
Van Haute, L., De Block, G., Liebaers, I., Sermon, K. & De Rycke, M. Generation of lung epithelial-like tissue from human embryonic stem cells. Respir. Res. 10, 105 (2009).
Yamamoto, Y. et al. Long-term expansion of alveolar stem cells derived from human iPS cells in organoids. Nat. Methods 14, 1097–1106 (2017).
Nikolić, M. Z. & Rawlins, E. L. Lung organoids and their use to study cell-cell interaction. Curr. Pathobiol. Rep. 5, 223–231 (2017).
Miller, A. J. & Spence, J. R. In vitro models to study human lung development, disease and homeostasis. Physiology 32, 246–260 (2017).
Clevers, H. Modeling development and disease with organoids. Cell 165, 1586–1597 (2016).
Hannan, N. R. F., Segeritz, C.-P., Touboul, T. & Vallier, L. Production of hepatocyte-like cells from human pluripotent stem cells. Nat. Protoc. 8, 430–437 (2013).
Gjorevski, N. et al. Designer matrices for intestinal stem cell and organoid culture. Nature 539, 560–564 (2016).
Bartfeld, S. & Clevers, H. Organoids as model for infectious diseases: culture of human and murine stomach organoids and microinjection of Helicobacter pylori. J. Vis. Exp. 2015, e53359 (2015).
Acknowledgements
Research reported in this publication was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number R01HL119215 to J.R.S. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
A.J.M., B.R.D. and J.R.S. conceived the studies. A.J.M., B.R.D., D.F.-T., D.R.H. and A.W.O. performed the experiments. A.J.M., B.R.D., D.F.-T., A.W.O., L.D.S. and J.R.S. analyzed the results. A.J.M. and J.R.S. wrote the manuscript. A.J.M., B.R.D., D.F.-T., A.W.O., D.R.H., L.D.S. and J.R.S. read and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
J.R.S., B.R.D. and A.J.M. hold patents pertaining to the lung organoid technologies described. The other authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Miller, A. J. et al. Stem Cell Rep. 10, 101–119 (2018): https://doi.org/10.1016/j.stemcr.2017.11.012
Dye, B. R. et al. eLife 5, e19732 (2016): https://doi.org/10.7554/eLife.19732
Dye, B. R. et al. eLife 4, e05098 (2015): https://doi.org/10.7554/eLife.05098
Supplementary information
Rights and permissions
About this article
Cite this article
Miller, A.J., Dye, B.R., Ferrer-Torres, D. et al. Generation of lung organoids from human pluripotent stem cells in vitro. Nat Protoc 14, 518–540 (2019). https://doi.org/10.1038/s41596-018-0104-8
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-018-0104-8
This article is cited by
-
Lung Organoids: Systematic Review of Recent Advancements and its Future Perspectives
Tissue Engineering and Regenerative Medicine (2024)
-
From cells to organs: progress and potential in cartilaginous organoids research
Journal of Translational Medicine (2023)
-
The interplay of cells, polymers, and vascularization in three-dimensional lung models and their applications in COVID-19 research and therapy
Stem Cell Research & Therapy (2023)
-
Linking SARS-CoV-2 to the circadian clock
Nature Cell Biology (2023)
-
Gradient to sectioning CUBE workflow for the generation and imaging of organoids with localized differentiation
Communications Biology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.