Abstract
The plasma membrane is enriched for receptors and signaling proteins that are accessible from the extracellular space for pharmacological intervention. Here we conducted a series of CRISPR screens using human cell surface proteome and integrin family libraries in multiple cancer models. Our results identified ITGAV (integrin αV) and its heterodimer partner ITGB5 (integrin β5) as the essential integrin α/β pair for cancer cell expansion. High-density CRISPR gene tiling further pinpointed the integral pocket within the β-propeller domain of ITGAV for integrin αVβ5 dimerization. Combined with in silico compound docking, we developed a CRISPR-Tiling-Instructed Computer-Aided (CRISPR-TICA) pipeline for drug discovery and identified Cpd_AV2 as a lead inhibitor targeting the β-propeller central pocket of ITGAV. Cpd_AV2 treatment led to rapid uncoupling of integrin αVβ5 and cellular apoptosis, providing a unique class of therapeutic action that eliminates the integrin signaling via heterodimer dissociation. We also foresee the CRISPR-TICA approach to be an accessible method for future drug discovery studies.
Similar content being viewed by others
Main
The plasma membrane is a semipermeable barrier that encloses intracellular components from the extracellular environment. In addition to the phospholipid bilayer, proteins are estimated to constitute as much as 50% of plasma membrane biomass. The cell surface proteome can be divided into integral membrane proteins (transmembrane proteins) and peripheral membrane proteins (membrane-anchored and other cell-surface-associated proteins), which are highly enriched for proteins involved in cellular adhesion, migration, communication, ligand binding, signal transduction, nutrition/ion transport and immunity1,2. An estimated 543 to 1,100 different proteins are present on the cancer cell surface3, many of which have functions that could influence disease progression and therapeutic response. Owing to the extracellular accessibility and the substantial biological functions of plasma membrane proteins, the cell surface proteome represents a valuable pool of targets for pharmacological intervention4,5.
Among these potential targets are integrins, a family of cell surface transmembrane receptors that have important roles in cell-to-cell and cell-to-extracellular-matrix (ECM) interactions6. Activation of integrins also controls the morphology, polarity and migration of cells by engaging the cells to the extracellular environments and by rearranging intracellular cytoskeleton components such as actin filaments (that is, outside-in signaling)7. Integrins can also be activated through their carboxyl terminus intracellular tails to engage extracellular ligands (that is, inside-out signaling)8. Thus far, 24 distinct integrins have been documented, in four subfamilies (the RGD receptors, collagen receptors, laminin receptors and leukocyte-specific receptors), each of which is a cell surface heterodimer comprising of one of the 18 α subunits and one of the eight β subunits in the human genome6. These diverse integrin α/β pairs govern tissue morphogenesis, homeostasis, angiogenesis, thrombosis and inflammatory response6,9. In addition, integrins including α4β1, α5β1, αVβ3 and αVβ5 have been implicated in the carcinogenesis and metastasis of various tumor types10. Therefore, pharmacological targeting of specific integrin α/β pairs has become an attractive field for therapeutic development9,11.
CRISPR–Cas9 (clustered regularly interspaced short palindromic repeats and CRISPR-associated endonucleases)12,13,14 gene suppression screens are powerful genetic approaches for identifying effector genes in biological systems15,16,17. For example, the DepMap consortium (https://depmap.org/portal/; BROAD Institute) has performed genome-wide CRISPR library knockout screens in ~1,000 cell line models and enabled the discovery of a wide range of cancer-cell-dependent genes18,19. Furthermore, recent advances in high-density CRISPR gene tiling have revealed the utility of CRISPR technology in protein domain and subdomain characterization20,21,22. The saturation CRISPR mutagenesis screen thus provides a powerful platform for examining critical functional areas within the protein of interest and could instruct discovery of therapeutics.
In this study, we conducted a series of unbiased CRISPR screens, including a surface proteome library screen and an integrin-family-focused library screen. As a result, we identified a requirement for integrin αV (ITGAV) and integrin β5 (ITGB5) in tumor cell maintenance. We also exploited the power of high-density CRISPR gene-tiling screens23,24,25,26,27,28 and developed a drug discovery tool named CRISPR-TICA (CRISPR-Tiling-Instructed Computer-Aided). This workflow allowed us to identify an integrin αVβ5 disruptor that targets the CRISPR-hypersensitive pocket within the β-propeller domain of ITGAV to prevent its interaction with ITGB5, providing a unique class of integrin inhibitor that acts through dissociation of the α/β heterodimers.
Results
Cell surface proteome CRISPR library screens
To identify critical cell surface proteins required for cancer cell expansion, we evaluated a mass-spectrometry-derived cell surface protein atlas (1,492 genes)4 together with a membrane protein database (2,418 genes)29 and the Human Protein Atlas – Plasma Membrane (2,254 genes; https://www.proteinatlas.org) and summarized 581 core cell surface proteins (Supplementary Table 1). Based on this, we developed a focused CRISPR library targeting the 581 genes encoding these cell surface proteins (involved in cellular adhesion, migration, communication, ligand binding, signal transduction, nutrition/ion transport, immunity and so on) with 2,905 single-guide RNAs (sgRNAs) (Fig. 1a; five sgRNAs per gene). We also spiked in a panel of 41 negative control sgRNAs (targeting nonhuman genes such as Luc, LacZ, Ren and Rosa26 and scrambled sequences) and 27 positive control sgRNAs (targeting cancer-essential genes such as MYC, PCNA and RPA3) (Extended Data Fig. 1a,b and Supplementary Table 2). We then delivered this library into five Cas9-expressing human cancer cell lines (MDA231, PANC1, U251, SW620 and H661) using lentiviral transduction and compared the change in frequency of each integrated sgRNA construct in these cells between day 0 and day 24 using high-throughput sequencing followed by the MAGeCK algorithm30 (Fig. 1b and Supplementary Table 3). Combined analyses revealed that in addition to proteins commonly required for cell proliferation (positive controls; yellow dots), these CRISPR screens identified ITGAV (encoded by the ITGAV gene) as the top essential cell surface protein in multiple cells representative of cancer types (Fig. 1c and Supplementary Table 3; additional analyses of these surface proteome screens are shown in Extended Data Fig. 2).
a, Schematic outline of cell surface proteome CRISPR screens (2,973 sgRNAs) in Cas9-expressing cancer cell models. b,c, Gene rankings for cell surface proteome CRISPR screens in five individual cell models (b) and the combined analysis (c) as calculated by the MAGeCK algorithm. The rankings of ITGAV (red), positive controls (yellow), negative controls (green) and the total library (gray) are indicated. d, Western blot of ITGAV and β-actin in MDA231-Cas9+ cells transduced with sgCtrl (n = 2 independent sgRNA sequences) and sgITGAV (n = 3 independent sgRNA sequences) for 3 days. e, Growth competition assay of MDA231-Cas9+ and PANC1-Cas9+ cells transduced with RFP-labeled sgCtrl (gray lines; two independent sgRNA sequences) and sgITGAV (red lines; three independent sgRNA sequences). Asterisk indicates that all three sgITGAV groups were significantly different (P < 0.01) from the two sgCtrl groups (n = 3 for each group). f,g, Cellular apoptosis detected by Annexin V+/DAPI− (f) and cell cycle monitored by EdU incorporation (g) in MDA231-Cas9+ cells transduced with sgCtrl and sgITGAV for 3 days (n = 3 for each group). h, Survival curves for cancer patients with high (top quartile; n = 927) versus low (bottom quartile; n = 927) ITGAV expression (data source: GEPIA). Data are presented as the mean ± s.e.m. P values were calculated by two-sided Student’s t-test. FC, fold change; GBM, glioblastoma multiforme.
To validate the library screen results, we transduced the Cas9+ MDA231 and PANC1 cells with sgRNAs targeting ITGAV (sgITGAV) to deplete the expression of endogenous ITGAV (Fig. 1d). Using a flow cytometric growth competition assay (Extended Data Fig. 3a), we found that cells transduced with sgITGAV were outcompeted by cells transduced with sgRNA targeting nonessential sequences (sgCtrl) (Fig. 1e), and further expression of an exogenous ITGAV complementary DNA (cDNA) rescued the cells from sgITGAV (Extended Data Fig. 4). CRISPR depletion of ITGAV in MDA231-Cas9+ cells also led to pronounced apoptosis (Fig. 1f) and arrested the cell cycle (Fig. 1g). Clinically, we found that ITGAV was overexpressed in multiple cancer types, including those tested in our cell surface proteome CRISPR screens (Supplementary Fig. 1). We also observed an association of high ITGAV expression with poor survival prognosis in patients with diverse cancer types (Fig. 1h; source: Gene Expression Profiling Interactive Analysis (GEPIA), including breast carcinoma, pancreatic adenocarcinoma, lung adenocarcinoma, hepatocellular carcinoma and glioma; total: ~3,700 patients)31, highlighting the requirement for ITGAV in cancer progression.
ITGAV mediates RAC1 signaling and F-actin assembly
Integrins are known to control cytoskeletal rearrangement via plasma-membrane-associated Rho family small GTPases, including RHOA, RAC1 and CDC42 (ref. 32). To elucidate the cytoskeletal signaling pathways affected by depletion of ITGAV, we analyzed the DepMap genome-wide CRISPR screen consortium database (https://depmap.org/portal/; BROAD Institute) and found higher correlations of CERES scores (CERES is a computational method to estimate gene-dependency levels from CRISPR–Cas9 essentiality screens)18 between ITGAV and RAC1 in the 769 tested cell models (Fig. 2a, purple, and Extended Data Fig. 5a; Pearson coefficient = 0.482; rank 6 of 17,709 genes) than between ITGAV and CDC42 or RHOA (green). The codependency relationship between ITGAV and RAC1 indicate that ITGAV primarily signals through RAC1 to mediate the intracellular response. We then performed RNA sequencing and gene set enrichment analysis (GSEA)33 on MDA231-Cas9+ cells transduced with sgCtrl versus sgITGAV. According to this transcriptomic analysis, the ‘RAC1_GTPase_Cycle’ was among the most depleted gene sets following sgITGAV transduction (Fig. 2b and Supplementary Table 4), supporting the data shown in Fig. 2a. Similar to sgITGAV, depletion of RAC1 by CRISPR (Fig. 2c) suppressed proliferation and survival of MDA231-Cas9+ cells (Fig. 2d–f). To examine the impact of ITGAV on cytoskeletal dynamics, we stained actin filaments (F-actin) with Alexa Fluor 488-conjugated phalloidin in MDA231-Cas9+ cells. Similar to sgRAC1 (purple), sgITGAV transduction (red) resulted in altered cellular morphology, disassembled cytoskeleton and reduced cell size (Fig. 2g,h). Overall, our results indicate an essential role of ITGAV/RAC1 signaling in cancer cell maintenance.
a, Gene ranking based on the Pearson coefficient (r) of CERES scores between ITGAV and RAC1 (purple) compared with CDC42 and RHOA (green) in the 769 tested cell models (Extended Data Fig. 5a). b, RNA sequencing analysis and GSEA showing changes in expression of the ‘RAC1_GTPase_Cycle’ gene set in MDA231-Cas9+ cells transduced with sgCtrl and sgITGAV for 3 days (n = 3 independent sgRNA sequences per group). c, Western blot of RAC1 and β-actin in MDA231-Cas9+ cells transduced with sgCtrl (n = 2 independent sgRNA sequences) and sgRAC1 for 3 days (n = 3 independent sgRNA sequences). d, Growth competition assay of MDA231-Cas9+ cells transduced with RFP-labeled sgCtrl (gray lines; two independent sgRNA sequences) and sgRAC1 (purple lines; three independent sgRNA sequences). Asterisk indicates that all three sgRAC1 groups were significantly different (P < 0.01) from the two sgCtrl groups (n = 3 for each group). e,f, Cellular apoptosis as detected by Annexin V+/DAPI− (e) and cell cycle monitored by EdU incorporation (f) in MDA231-Cas9+ cells transduced with sgCtrl and sgRAC1 for 3 days (n = 3 for each group). g, Representative fluorescence images of F-actin (fluorescein isothiocyanate (FITC), green) and nucleus (DAPI, blue) staining in MDA231-Cas9+ cells transduced with sgCtrl, sgITGAV and sgRAC1. Scale bars, 20 µm. h, Violin plot showing distribution of cell size (µm2) in MDA231-Cas9+ cells transduced with sgCtrl, sgITGAV and sgRAC1. Data are presented as the mean ± s.e.m. P values were calculated by two-sided Student’s t-test. NS, not significant.
Integrin family CRISPR screens highlighted integrin αVβ5
Integrins are obligate heterodimeric cell surface receptors composed of one of the 18 α subunits and one of the eight β subunits in the human genome (Fig. 3a)6. To pinpoint the critical components within the integrin family that collaborate with ITGAV for cancer cell maintenance, we developed another CRISPR library targeting the gene coding regions of each of the 26 integrin subunits with 25 sgRNAs per gene (Extended Data Fig. 1c and Supplementary Table 2; total 650 sgRNAs plus control sgRNAs) for a CRISPR depletion screen in the Cas9+ MDA231 and PANC1 cells (Fig. 3b). These validation screens suggested that in addition to those targeting ITGAV, the sgRNAs targeting ITGB5 were strongly depleted in both cancer cell models (Fig. 3c and Supplementary Fig. 2). We then annotated the CRISPR impact score (the median log10 fold change of the 25 sgRNAs for each subunit) to the integrin heterodimer network (a total of 24 different integrins, each with a unique α/β combination)6 and identified integrin αVβ5 (an RGD receptor mediating cell-to-ECM interaction) as the top essential integrin pair in these cancer cells (Fig. 3d, red dotted circle). CRISPR depletion of ITGB5 (but not the other ITGAV heterodimer partners, including ITGB1/3/6/8) suppressed proliferation of MDA231 cells (Fig. 3e). Similar to the results for sgITGAV, depletion of ITGB5 (Fig. 3f) impaired the survival and cell cycle of MDA231 cells (Fig. 3g,h), phenocopying the effect of sgITGAV (Fig. 1f,g). Further analysis of gene codependency in the DepMap genome-wide CRISPR screen consortium database showed that the highest correlation of CERES scores in the 769 tested cell models was that between ITGAV and ITGB5 (Fig. 3i, blue; Extended Data Fig. 5b; Pearson coefficient = 0.686; rank 1 of 17,709 genes). By contrast, the correlations of CERES scores between ITGAV and other partner β subunit coding genes (ITGB1/3/6/8) were much weaker (Fig. 3i, yellow; Extended Data Fig. 5b), suggesting a selective requirement for the integrin αVβ5 heterodimer in cancer cell expansion.
a, Model of integrin α (red) and β (blue) subunits and domain structures. The binding site of the extracellular ligand (yellow) is assembled upon heterodimerization of the α/β subunits. b, Schematic outline of integrin family CRISPR screens (712 sgRNAs) in Cas9-expressing MDA231 and PANC1 cells. c, Fold change of each sgRNA from day 0 to day 24 in MDA231-Cas9+ (x axis) and PANC1-Cas9+ (y axis) cells. The sgRNAs targeting ITGAV (red dots), ITGB5 (blue dots), positive controls (yellow triangles), negative controls (green triangles) and the total library (gray dots) are indicated. d, Heatmap showing CRISPR impact scores (median log10 fold change of 25 sgRNAs) of each integrin subunit in the integrin network consisting of 24 distinct integrin α/β heterodimers. The solid lines indicate the integrin α/β pairs forming the RGD receptors (yellow), collagen receptors (pink), laminin receptors (brown) and leukocyte-specific receptors (green). The red dotted circle highlights αVβ5 as the top essential integrin heterodimer in cancer cells. e, Growth competition assay of MDA231-Cas9+ cells transduced with RFP-labeled sgCtrl (gray lines; two independent sgRNA sequences) and sgITGB1/3/5/6/8 (blue lines; three independent sgRNA sequences for each gene). Asterisk indicates that all three sgRNAs for each ITGB gene group were significantly different (P < 0.01) from the two sgCtrl groups (n = 3 for each group). f, Western blot of ITGB5 and β-actin in MDA231-Cas9+ cells transduced with sgCtrl (n = 2 independent sgRNA sequences) and sgITGB5 (n = 3 independent sgRNA sequences) for 3 days. g,h, Cellular apoptosis detected by Annexin V+/DAPI− (g) and cell cycle monitored by EdU incorporation (h) in MDA231-Cas9+ cells transduced with sgCtrl and sgITGB5 for 3 days (n = 3 for each group). i, Gene ranking based on the Pearson coefficient (r) of CERES scores between ITGAV and ITGB5 (blue) compared with other ITGAV partner β subunit genes ITGB1/3/6/8 (yellow) in the 769 tested cell models (Extended Data Fig. 5b). Data are presented as the mean ± s.e.m. P values were calculated by two-sided Student’s t-test.
CRISPR gene tiling pinpointed the β-propeller domain of ITGAV
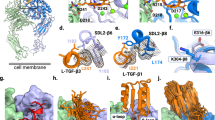
To define the regions of ITGAV required for cancer cell survival, we employed a high-density gene tiling scan that pinpoints the functional regions within a protein by CRISPR-induced mutagenesis23,24,25,26,27,28. For this, we constructed a CRISPR library of 348 sgRNAs that target every position with an NGG protospacer adjacent motif within the ITGAV coding exons (Fig. 4a, Extended Data Fig. 1d and Supplementary Table 2). We then utilized lentiviral transduction to deliver this library into the Cas9-expressing MDA231 cells and detected the frequencies of each sgRNA sequence before versus after 24 days of culture by NextSeq550 sequencing (Supplementary Table 5). After smoothen modeling of the local sgRNAs34, our CRISPR-tiling scan identified seven regions with critical roles (Fig. 4b, numbers 1–7) within the β propeller domain of ITGAV (dashed box; F31–A467). We then mapped the normalized CRISPR score (NCS) on a three-dimensional (3D) structure of ITGAV (Fig. 4c; AlphaFold ID: P06756)35. We found that the CRISPR-sensitive peptide regions within the β-propeller domain represented the tips of the seven blade-shaped structures facing toward the aromatic residue-enriched central cavity, designated ‘hypersensitivity-illustrated pocket’ or ‘HIP’ (Fig. 4d; including F51, W123, F189, Y254, F308, S372 and Y436), which is in direct contact with a basic amino acid (lysine or arginine) in the loop motif of the βA domain within the β integrin subunits36,37,38.
a, Schematic outline of the ITGAV high-density CRISPR-tiling scan (412 sgRNAs) in MDA231-Cas9+ cells. b, 2D annotation of ITGAV CRISPR scan. The red line indicates the smoothened model of NCS derived from 348 sgRNAs (dots) targeting the coding exons of ITGAV. The median NCS of the positive control (gray dotted line; defined as −1.0) and negative control (defined as 0) sgRNAs are highlighted. The brown dashed box contains the β-propeller domain. The numbers 1–7 pinpoint the CRISPR-hypersensitive regions within the β-propeller domain. c, 3D annotation of ITGAV CRISPR scan NCS relative to AlphaFold structural modeling of ITGAV (AlphaFold ID: P06756). d, Enlarged view of the β-propeller domain showing the CRISPR-hypersensitive regions (numbers 1–7 as indicated in b) pointing to the center cavity of the β-propeller HIP. The residues contributing to this aromatic-enriched pocket are highlighted. e, Schematic outline of the NanoBRET reporter system for detecting the ITGAV–ITGB5 interaction in living cells. f, Effect of alanine substitution of the ITGAV β-propeller HIP residues (brown; n = 3 for each group) on the NanoBRET signal compared with the wild-type ITGAV (gray; n = 3 for each group). Data are represented as mean ± s.e.m. P values were calculated by two-sided Student’s t-test. Ex, exon; TSS, transcription start site. TM, transmembrane.
To investigate the role of the β-propeller HIP in the ITGAV–ITGB5 interaction in living cells, we employed a bioluminescence resonance energy transfer (NanoBRET) assay (Fig. 4e)39,40 by fusing the energy donor NanoLuc luciferase to the cytoplasmic tail of ITGB5. We also fused the energy acceptor ‘HaloTag’ to the cytoplasmic tail of ITGAV. When adding the NanoLuc luciferase substrate (generating a 460 nm donor signal) and the HaloTag ligand (generating a 618 nm acceptor signal), this integrin αVβ5 reporter will turn on the NanoBRET signal (618 nm versus 460 nm ratio) while the ITGAV and ITGB5 subunits are proximal (Fig. 4e, left). Conversely, the NanoBRET signal will be abolished upon disengagement between the ITGAV and ITGB5 subunits (Fig. 4e, right). Alanine substitution of any one of the β-propeller HIP residues (F51A, W123A, F189A, Y254A, F308A, S372A or Y436A) on ITGAV attenuated the NanoBRET signal by 50% to 70% compared with wild-type ITGAV (Fig. 4f; alanine substitution of the aromatic residues outside of HIP are shown in Extended Data Fig. 6), indicating a pivotal role of the ITGAV β-propeller HIP in integrin αVβ5 assembly.
A lead compound that disrupts ITGAV heterodimerization
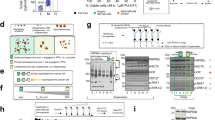
To identify additional classes of inhibitors that block ITGAV heterodimerization, we developed a CRISPR-TICA pipeline that enables de novo identification of small molecular compounds for binding to CRISPR-hypersensitive surface areas of the targeted protein. We reasoned that the CRISPR-hypersensitive surface areas (which cannot tolerate CRISPR-induced mutagenesis; Fig. 4d) might indicate critical functional positions amenable to pharmaceutical inhibition. We mapped the NCS on a crystal structure of the ITGAV β-propeller domain (Fig. 5a; PDB ID: 3IJE)41,42,43 and used AutoSite44 and AutoDock Vina45 to predict the binding affinity of ~128 K diverse compounds (collected by the National Cancer Institute/Developmental Therapeutics Program (NCI/DTP) Open Chemicals Repository) to the β-propeller HIP (Fig. 5a, box). We then selected the top 500 predicted binders (Fig. 5b and Supplementary Table 6; binding free energies (ΔG°) ≤ −11.6 kJ mol−1) for CellTiter Glo and CCK8 (Cell Counting Kit 8) validation screens in MDA231 cells (Fig. 5c and Supplementary Table 7; each compound tested at 10 µM) and identified nine candidate compounds that could suppress the viability of MDA231 cells with <10% viability in both assays (Fig. 5d and Extended Data Fig. 7).
a, 3D ‘docking box’ (cube) defined by the CRISPR-hypersensitive regions (numbers 1–7) within the ITGAV β-propeller domain. b, Compound (Cpd) ranking based on free binding energy (ΔG°) to the ‘docking box’ within the β-propeller domain predicted by AutoDock Vina. c,d, Heatmap showing relative CellTiter Glo (left) and CCK8 (right) signals (percentage of the signal for dimethyl sulfoxide; DMSO) in MDA231 cells incubated with 10 µM of 500 selected compounds (c) and the top nine effective compounds (d) for 3 days. Effective cell killing was defined as less than 10% relative signals for both CellTiter Glo and CCK8 assays. e, Schematic outline of flow cytometric measurement of cell surface integrin αVβ5 using a monoclonal antibody against integrin αVβ5 heterodimers. f, Effects of the top nine candidate compounds on cell surface integrin αVβ5 levels upon 1 h compound treatments (n = 4 for each condition). g,h, Cellular apoptosis detected by Annexin V+/DAPI− (g) and cell cycle monitored by EdU incorporation (h) in MDA231 cells treated with Cpd_AV2 (40 µM) for 0 to 3 h (n = 3 for each time point). i, Representative fluorescence images of F-actin (FITC, green) and nucleus (DAPI, blue) staining in MDA231 cells treated with control (DMSO) and Cpd_AV2 (40 µM) for 10 min. Scale bars, 20 µm. j, Violin plot showing the distribution of cell size (µm2) in MDA231 cells treated with control (DMSO) and Cpd_AV2 (40 µM) for 10 min. Data are presented as the mean ± s.e.m. P values were calculated by two-sided Student’s t-test.
As mutagenesis of the β-propeller HIP of ITGAV significantly affected the assembly of integrin αVβ5 (Fig. 4f), we utilized a flow cytometric assay to detect integrin αVβ5 dimers on the cell surface using an monoclonal anti-integrin αVβ5 heterodimer antibody (Fig. 5e and Extended Data Fig. 3b). This allowed us to monitor the rapid changes in integrin αVβ5 levels on the cell surface upon 1 h compound treatments. We found that most of the candidate compounds were unable to reduce cell surface integrin αVβ5 levels (Cpd_AV5/82/259/343/377/388/469) or exhibited a notable fluorescent background (Cpd_AV84), suggesting that the cell inhibitory effects of these compounds were not associated with integrin αVβ5 disruption (Fig. 5f, green). On the other hand, one of the nine candidate compounds (Cpd_AV2) exhibited potential to reduce the presence of integrin αVβ5 on the cell surface (Fig. 5f, orange; minimum inhibitory concentration ~40 µM with half-maximal inhibitory concentration (IC50) ~6.9 µM; additional validation data shown in Supplementary Fig. 3). At the cellular level, we found that Cpd_AV2 led to pronounced apoptosis and cell cycle arrest by 3 h posttreatment (Fig. 5g, h). Furthermore, Cpd_AV2 induced a drastic change in cell morphology, cytoskeleton assembly and cell size as early as 10 min posttreatment (Fig. 5i, j). These effects of Cpd_AV2 resembled those triggered by ITGAV depletion (Figs. 1f,g and 2g,h), marking Cpd_AV2 as the top integrin αVβ5 disruptor from the compound screen.
To validate the interaction between Cpd_AV2 and ITGAV, we purified the recombinant His6-tagged ITGAV [31–492 amino acids (aa)] from Escherichia coli (Fig. 6a; covers the β-propeller domain of ITGAV) and examined the protein thermal stability under control versus Cpd_AV2 conditions. We observed that incubation of Cpd_AV2 (40 µM) increased the melting temperature (Tm) from 52.0 °C to 58.1 °C (Fig. 6b; ΔTm = 6.1 °C), suggesting an interaction between Cpd_AV2 and the purified ITGAV β-propeller domain. Furthermore, molecular docking of the bound complexes demonstrated favorable interactions between Cpd_AV2 (yellow) and the ITGAV β-propeller central cavity (Fig. 6c) with a superior binding energy (ΔG° = −15.0 kJ mol−1; ranked in the top two of the ~128 K docked compounds), showing competitive binding of Cpd_AV2 against the ITGB5 βA loop (right panel, cyan fragment; specifically at the K287 position) on the surface of the ITGAV β-propeller domain. Molecular dynamics simulations starting from the AlphaFold2 (ref. 35) structural model of the ITGAV–ITGB5 complex showed that K287 of ITGB5 interacts closely with multiple aromatic residues in ITGAV (Extended Data Fig. 8a–d), thereby playing a critical part in holding the complex together. This interaction is broken by the presence of Cpd_AV2 (Extended Data Fig. 8e). Mutation of K287 to alanine significantly attenuated the ITGAV–ITGB5 NanoBRET signal (Extended Data Fig. 8f), highlighting an essential role of the ITGB5 K287 in integrin αVβ5 assembly. These analyses together provide mechanistic insights into the role of Cpd_AV2 in disrupting the ITGAV–ITGB5 interaction. Compared with Cpd_AV2, we found that cilengitide46,47,48 (an RGD-mimetic ITGAV inhibitor examined in multiple clinical trials) was unable to disrupt the level of cell surface integrin αVβ5 heterodimer (Fig. 6d) and resulted in less efficacy of cell suppression (Fig. 6e). Investigation of the effectiveness of Cpd_AV2 against commonly used cancer cell models indicated a utility of Cpd_AV2 for pan-cancer treatment (Fig. 6f; IC50 = 1.3–5.2 µM in cell cultures). Collectively, our data suggest that Cpd_AV2 (NCI/DTP NSC identifier: 268394; IUPAC name: (5S,7R,12S,14S)-5,14-dimethyl-7,12-dinaphthalen-2-yl-1,4,8,11-tetrazacyclotetradecane; structure shown in Fig. 6g) acts through disrupting the ITGAV heterodimerization to eliminate integrin αVβ5 signaling, providing an alternative and potentially more potent ITGAV-targeted therapeutic approach compared with traditional RGD-blockers (Fig. 6h).
a, Purification of bacterial-expressed recombinant ITGAV β-propeller domain (peptide region 31–492 aa; N-terminal His6-tagged) using immobilized metal affinity chromatography (IMAC) and anion exchange chromatography (IEX). The input and purified ITGAV β-propeller domain samples were visualized by gel electrophoresis and silver staining (right; gel representative of two independent protein purification experiments). b, Protein thermal stability as estimated by fluorescent dye incorporation of the purified ITGAV β-propeller domain under control (DMSO) and Cpd_AV2 (40 µM) conditions. c, Protein surface model (left) showing a docking simulation of the ITGAV β-propeller domain (colored by NCS) interacting with Cpd_AV2 (yellow). Protein ribbon model (right) illustrates an overlap of ITGB5 lysine 287 (within βA loop; cyan) and Cpd_AV2 (yellow) binding on the β-propeller HIP of ITGAV. d,e, Effects of cilengitide and Cpd_AV2 on cell surface integrin αVβ5 levels after 1 h treatment (d) and cell expansion after 72 h treatment in MDA231 cells (e) (n = 3 for each group). f, Effects of 72 h Cpd_AV2 treatment on expansion of six cancer cell models (n = 3 for each group). g, Chemical structure of Cpd_AV2 (source: NCI/DTP Open Chemicals Repository). h, Model showing distinct mechanisms of action between Cpd_AV2 (left) and cilengitide (right) for suppressing ECM-to-integrin αVβ5 signaling (middle). Data are presented as the mean ± s.e.m. P values were calculated by two-sided Student’s t-test. NSCLC, nonsmall-cell lung cancer.
Discussion
The cell surface proteome is enriched for structural and signaling components that mediate diverse biological activities under normal and disease conditions1,2,3,4. A better understanding of the cell surface protein genes related to cancer progression could provide new therapeutic opportunities and shed light on novel mechanisms of drug action. In this study, we performed a series of CRISPR genetic screens (that is, a cell surface proteome screen, an integrin family screen and a high-density ITGAV CRISPR-tiling screen) in multiple cancer cell models. Using these functional genetics approaches, we identified the critical role of integrin αVβ5 in cancer cell maintenance. We also demonstrated that the CRISPR-hypersensitive cavity within the β-propeller of ITGAV is indispensable to integrin αVβ5 heterodimerization, offering a therapeutic pocket for pharmacological development.
ITGAV (also known as CD51) is expressed in multiple tissue cell types and is involved in tissue developmental steps including angiogenesis49,50. The ITGAV integrins (αVβ1, αVβ3, αVβ5, αVβ6, αVβ8) are heterodimeric cell surface receptors that recognize RGD-containing ECM proteins (for example, vitronectin, fibronectin, osteopontin, von Willebrand factor and thrombospondin)6 and intercellular signaling molecules (for example, TGFβ)51,52. Serving as the primary receptors for vitronectin (offered to cells under standard cell culture conditions for cell-to-plate adhesion), integrins αVβ3 and αVβ5 are known to protect tumor-derived cells from apoptosis53,54. Increased expression of ITGAV has been reported as a prognostic marker in diverse cancer types (breast cancer, prostate cancer, ovarian cancer, glioblastoma, myeloma, hepatocellular carcinoma, skin carcinoma, colorectal adenocarcinoma, esophageal adenocarcinoma, pancreatic adenocarcinoma and so on)9,10,48,55,56,57,58,59,60,61,62. A recent study also demonstrated the role of integrin αVβ5 in Zika virus entry to glioblastoma stem cells63. Genetic suppression of ITGAV was shown to impair the proliferation, survival and migration of cancer cells, suggesting that ITGAV could serve as a therapeutic target to inhibit tumor progression and metastasis11. Owing to the potential of ITGAV inhibition in the therapeutics market, pharmacological targeting of ITGAV integrins has been widely explored over the past 20 years, with more than 30 inhibitors currently under clinical and preclinical development64. Nonetheless, the efficacy of current ITGAV-targeted therapies remains elusive, emphasizing the need for a novel and perhaps more effective blockade for ITGAV signaling.
Thus far, ITGAV inhibitors have been primarily focused on blocking interactions between heterodimerized ITGAV integrins (for example, αVβ3 and αVβ5) and RGD-containing ECM proteins9,10. Specifically, the current ITGAV integrin inhibitors belong to two principal strategies: there are RGD-mimetic molecules (for example, cilengtide, GLPG0187 and MK-0429) and ITGAV-integrin-specific blocking antibodies (intetumumab, etaracizumab, abituzumab (EMD 525797) and so on)11,64,65. Although it prevents the anchorage of cancer cells to ECM proteins, the most advanced compound among these traditional integrin inhibitors (cilengitide) showed limited benefits in several animal and human trials66,67,68, potentially owing to incomplete suppression of basal integrin signaling, as the α/β heterodimers remained undisrupted (Fig. 6h, right). On the other hand, no compounds that disrupt the interactions between ITGAV and its partner β subunits have previously been reported. Our results highlight a class of inhibitory mechanism that dissociates the integrin αVβ5 by blocking the CRISPR-hypersensitive β-propeller pocket (which cannot tolerate CRISPR-induced mutagenesis; Fig. 4d) in ITGAV. This strategy provides an additional and perhaps more effective therapeutic action by eliminating basal integrin heterodimer signaling (Fig. 6h, left). Furthermore, this drug action might also prevent inside-out integrin activation6,8 from attenuating therapeutic efficacy.
Structurally, the basic amino acid encapsulated in the β-propeller of ITGAV is conserved across the ITGB1/3/5/6/8 peptides (Extended Data Fig. 9a; K/R287), highlighting the potential of Cpd_AV2 to disrupt the functions of αVβ1, αVβ3, αVβ5, αVβ6 and αVβ8 integrins. As a proof of concept, we monitored the integrin-αVβ6-dependent cell adhesion of HT-29 colorectal carcinoma cells to fibronectin reported by ref. 69. We found that preincubation of the HT-29 cells with Cpd_AV2 led to dose-dependent blockade of HT-29 cell adhesion to the fibronectin-coated wells (Extended Data Fig. 9b). We foresee that this integrin-targeting strategy will also be applicable to other integrin α subunits for compounds binding to their specific β-propeller pockets (a common feature within the integrin α subunits). Notably, the function of integrins is heavily influenced by the glycosylation of their extracellular domains70. In addition to the in vitro protein/compound biochemical assays that utilize nonglycosylated bacterially expressed proteins (Fig. 6a,b), cell-based characterizations such as cell surface flow cytometry and the NanoBRET interaction assay (Fig. 5f and Supplementary Fig. 3) in mammalian cells are necessary to validate the impact of compounds on the full-length glycosylated integrins.
High-throughput CRISPR library screens have been performed in diverse cancer cell types, revealing critical mechanisms mediating tumorigenesis and therapeutic response15,16,17,18,19. By contrast, the potential of CRISPR technology to investigate gene function at subgene (that is, protein domain or motif) resolution is now being explored20,21,22. For example, high-resolution CRISPR gene tiling screens have been used to identify the essential elements within catalytic core domains22,24,26. In addition, CRISPR tiling has the sensitivity to pinpoint protein–protein interaction sites within screened proteins21,25,28. Our CRISPR gene scan has also been used to identify a protein domain mediating oncoprotein nuclear trafficking27. In the present study, we further exploited the utility of high-density CRISPR gene tiling to identify a protein surface pocket for therapeutic development (Figs. 4 and 5). We propose that CRISPR-hypersensitive surface areas (that is, those that cannot tolerate CRISPR-induced mutagenesis) might correspond to critical positions that, when targeted by small molecules, could disrupt the normal function of the protein. To validate this hypothesis, we obtained previously published CRISPR-tiling data from ref. 23 and identified four proteins with well-defined inhibitors targeting their CRISPR-hypersensitive pockets (Extended Data Fig. 10; including the bromodomain of BRD4 and the kinase catalytic cores of AURKB, CDK1 and WEE1)71,72,73,74. These analyses demonstrated the utility of CRISPR tiling as a generalizable approach for future drug discovery.
In short, our ITGAV CRISPR-tiling scan offered ~3.0 aa per sgRNA resolution (Fig. 4a, b) and clearly distinguished the CRISPR-hypersensitive central pocket from the surrounding β-propeller domain (Fig. 4d). Notably, this 3D pocket was assembled from seven discontinuous CRISPR-hypersensitive segments (Fig. 4b, labeled 1–7; separate from each other in their two-dimensional (2D) peptide positions) in the ITGAV β-propeller domain, highlighting the capacity of the CRISPR-tiling scan for subdomain functional recognition beyond traditional domain mapping. This finding prompted us to develop the CRISPR-TICA workflow for de novo compound discovery and enabled us to identify a lead inhibitor (Cpd_AV2) that disrupts integrin heterodimerization.
Methods
Cell lines and cell culture
HEK293, PANC1 and SW620 cells were obtained from the American Type Culture Collection; MDA231 (that is, MDA-MB-231) cells were obtained from M. Feng (City of Hope Cancer Center); H661 cells were obtained from J. Qi (Dana Farber Cancer Institute); and U251 cells were obtained from M. Chen (City of Hope Cancer Center). Cells were cultured in Dulbecco’s modified Eagle medium (DMEM; Gibco) supplemented with 10% fetal bovine serum (Omega Scientific). All media were supplemented with penicillin (100 units per ml; Gibco), streptomycin (100 μg ml−1; Gibco), l-glutamine (2 mM; Gibco) and plasmocin (0.5 μg ml−1; InvivoGen). All cells were cultured in a 37 °C incubator with 5% CO2. Cells stably expressing the Cas9 endonuclease were established via transduction of LentiCas9-Blast (52962, Addgene) lentivirus and selected using blasticidin (Gibco). For assay preparation, all adherent cells were removed from plates with a nonenzymatic cell dissociation buffer (13150016, Gibco).
CRISPR library and single sgRNA cloning
Briefly, guide RNA oligos were synthesized by microarray (CustomArray; for library cloning) or individual oligosynthesis (IDT; for single sgRNA) and cloned into the ipUSEPR lentiviral sgRNA vector (hU6-driven sgRNA coexpressed with EF-1a-driven red fluorescent protein (RFP) and puromycin-resistance gene) using the BsmBI (NEB) restriction sites (Extended Data Fig. 1a). CRISPR sgRNAs were selected using the BROAD Institute Genetic Perturbation Platform – CRISPick17. For the cell surface proteome CRISPR library, 2,905 sgRNA sequences targeting 581 genes encoding cell surface proteins were designed (Extended Data Fig. 1b; five sgRNAs per gene). For the integrin family CRISPR library, 650 sgRNA sequences targeting 26 genes encoding integrin subunits were selected (Extended Data Fig. 1c; 25 sgRNAs per gene). For the ITGAV tiling scan CRISPR library, 348 sgRNA sequences targeting every protospacer adjacent motif within the human ITGAV coding exons were covered (Extended Data Fig. 1d; 9 bp per sgRNA). The cloned libraries were first sequenced with a NextSeq to ensure at least 90% of the sgRNA sequences exhibited a minimal ten reads per million reads. Quality control sequencing reports for these libraries are shown in Supplementary Table 2. The sequences of single sgRNAs selected for validation experiments are listed in Supplementary Table 8.
Lentiviral production and transduction
Lentiviruses were produced in HEK293 cells (CRL-1573, American Type Culture Collection) with packaging plasmids pPAX2 (12260, Addgene) and pMD2.G (12259, Addgene). Then, pPAX2, pMD2.G and a lentiviral backbone plasmid were mixed in a 1:1:1 ratio in Opti-MEM medium (31-985-062, Gibco) in the presence of 50 µg ml−1 polyethyleneimine (PRIME-P100-100MG, Serochem LLC). Twenty-four hours after transfection of HEK293 cells, the medium supernatant was aspired and replaced with fresh DMEM. Then, the transfected cells were allowed to grow for 48 h to produce lentiviruses. Subsequently, the virus-containing supernatants were incubated with 10% polyethylene glycol (BP233-1, ThermoFisher Scientific) at 4 °C overnight and then centrifuged at 3,000g, 4 °C, 30 min, to collect precipitated viral particles. After that, the viral pellets were resuspended with appropriate DMEM, aliquoted and kept at −80 °C.
CRISPR library screens
The CRISPR library screens were performed as previously described28. Briefly, the CRISPR sgRNA libraries were delivered to Cas9-expressing cells using lentiviral infection (~15% transduction rate, monitored based on RFP expression). To achieve 1,000× coverage of the library in each screen, 30 million cells for the cell surface proteome library screen, six million cells for the integrin family library screen and four million cells for the ITGAV high-density CRISPR-tiling scan were used to start each screen replicate. The library-infected cells were then selected using puromycin (1.5 μg ml−1; Gibco) and subcultured every 3 days. The integrated sgRNA at the start (day 0) and end (day 24) timepoints was amplified by PCR (NEBNext Ultra II Q5; NEB) using the previously reported DCF01 5′-CTTGTGGAAAGGACGAAACACCG-3′ and DCR03 5′-CCTAGGAACAGCGGTTTAAAAAAGC-3′ primers22. After sequencing with a NextSeq550 (Illumina), the read count of each 20 nucleotide sequence that matched an sgRNA in the library of guide RNA sequences was calculated. For the cell surface proteome screen, essential genes were identified using MAGeCK analysis30. For the integrin family gene panel screen, the CRISPR impact score was defined as the median log10 fold change of the 25 sgRNAs for each integrin subunit encoding gene. For the ITGAV CRISPR gene tiling scan, the NCS indicated the frequency change of each sgRNA between the start and end of the screen on a log10 scale, where the median score of the negative control sgRNA (defined as 0; sgRNA targeting nonessential sequences) and the median score of the positive control sgRNA (defined as −1.0; sgRNA targeting MYC, BRD4, RPA3, PCNA and so on) were obtained from the control sgRNAs within the screen libraries. Low-frequency sgRNAs (below 5% of the expected frequency) in the library were removed from the analysis.
Annotation of CRISPR gene tiling scan
The ITGAV CRISPR gene tiling scan library (348 sgRNAs targeting ITGAV coding exons) was delivered into the MDA231-Cas9+ cells and processed using the methods described above. For 2D annotation, the NCSs of individual sgRNAs were processed by Gaussian kernel smoothing in R22, and the average score over the trinucleotide codons was calculated for each peptide position. For 3D annotation, we first obtained 3D structure data for ITGAV from the AlphaFold Protein Structure Database (Protein ID: P06756)35 and the Research Collaboratory for Structural Bioinformatics Protein Data Bank (RCSB PDB ID: 3IJE)41,42,43. Subsequently, the smoothened ITGAV CRISPR NCSs (from the 2D annotation) were mapped onto the ITGAV 3D structures using the ‘Defined Attribute’ and ‘Render by Attribute’ functions in UCSF Chimera 1.15 (ref. 75).
CRISPR-TICA workflow
The human ITGAV β-propeller structure was extracted from 3IJE using PyMOL v.2.0.4 (Schrödinger, LLC) and the PDB 2PQR server76, and the resultant pqr file was converted into pdbqt format using AutoDockTools77. The space within the CRISPR-hypersensitive region suitable for compound binding (the docking box shown in Fig. 5a) was suggested by AutoSite44. The 3D chemical structure of ~128 K diverse compounds (collected in the NCI/DTP Open Chemicals Repository; https://dtp.cancer.gov) downloaded as mol2 files were split into subsets of 20,000 compounds using Open Babel v.2.4.1 (ref. 78). Subsequently, each subset was converted into pdbqt format (the input file format for AutoDock Vina) using PyRx v.0.9.7 (ref. 79). Having prepared both ligand and protein structures for structure-based drug discovery, we used AutoDock Vina v.1.1.2 (ref. 45), an in silico molecular docking program, to virtually dock these compounds into the defined docking box using the City of Hope Saturn 2 Linux cluster. Finally, the docking data were processed and exported to csv files using Raccoon2 (ref. 77).
Cell-based survival screen using CellTiter Glo and CCK8 assays
The top 500 ITGAV β-propeller binders suggested by CRISPR-TICA were requested from the NCI/DTP Open Chemicals Repository for functional validation. Compound information is listed in Supplementary Table 6. MDA231 cells were seeded at 10,000 cells per well in 96-well plates for 24 h, and the compounds were added to a final concentration of 10 µM for another 72 h. For the Cell Counting Kit 8 (CCK8) assay, 10 µl of CCK8 reagent (K1018, APExBio) was added to cells (100 µl per well), followed by incubation at 37 °C for 1 h, and the absorbance at 450 nm was measured using an Infinite M1000 Pro plate reader (Tecan Trading AG). For the CellTiter Glo assay, cells were washed twice with phosphate-buffered saline (PBS) and resuspended by trypsinization. The resuspended cells (50 µl) were mixed with CellTiter Glo 2.0 reagent (10 µl; G9241, Promega) in white flat-bottomed 96-well plates (353296, Corning) at room temperature for 10 min, and the luminescence was measured using an Infinite M1000 Pro plate reader (Tecan Trading AG). The relative CellTiter Glo (%) and CCK8 (%) signals were normalized to the control condition (DMSO).
Western blotting
Cells were harvested and lysed in 1% sodium dodecyl sulfate (SDS) lysis buffer (1% SDS, 50 mM Tris pH 7.5), and the proteins were denatured at 95 °C for 15 min. Protein concentration was measured using a DC Protein Assay Kit II (5000112, Bio-Rad). Denatured protein samples were separated on Bolt 4–12% Bis-Tris plus gels (NW04125, Invitrogen) or 3–8% Tris-acetate gels (EA0375, Invitrogen) using electrophoresis. The separated protein bands were transferred onto polyvinylidene fluoride (PVDF) Mini Stacks (0.2-μm pore size; IB24002, Invitrogen) using an iBlot 2 (Invitrogen). PVDF membranes were blocked with 5% bovine serum albumin (Fisher Scientific) in Tris-buffered saline with Tween-20 (TBST) at room temperature for 1 h and then probed with primary antibodies for ITGAV (4711, Cell Signaling Technology, 1:1000), ITGB5 (3629, Cell Signaling Technology; 1:1000), RAC1 (4651, Cell Signaling Technology; 1:1000) and β-actin (ab8226, Abcam; 1:5000) at 4 °C overnight. After the membranes had been washed with TBST three times, HRP-conjugated goat anti-mouse (31430, Invitrogen; 1:10,000) or goat anti-rabbit (31460, Invitrogen; 1:10,000) IgG secondary antibodies were added, followed by shaking at room temperature for 1 h. The washed PVDF membranes were then incubated with SuperSignal West Femto Substrate (P134095, ThermoFisher), and the chemiluminescence signals were detected using a ChemiDoc imaging system (Bio-Rad). The antibody concentrations are listed in Supplementary Table 10. The uncropped gel blot images are shown in Supplementary Fig. 4.
Flow cytometric assays
Flow cytometric data were collected on an Attune NxT flow cytometer with an autosampler (ThermoFisher Scientific). For the RFP-coupled growth competition assay, the ipUSEPR vector system, which expresses an sgRNA together with a TagRFP fluorescent protein, was used to infect the Cas9+ cells at a ~50% transduction rate. The percentage of cells with an RFP fluorescence signal (RFP+%) was normalized to the RFP+% on day 0 (48 h after lentiviral infection). The cell cycle was monitored by EdU incorporation (10 µM EdU at 37 °C for 2 h) using Click-iT Plus EdU Alexa Fluor 647 Assay Kits (C10634, Invitrogen). Apoptotic cells were detected based on the Annexin V+/DAPI− population using an Annexin V Apoptosis Detection Kit (50-112-9048, Invitrogen). Live cells were defined by exclusion of 4′,6-diamidino-2-phenylindole (DAPI; D1306, Invitrogen) DNA staining. Cell surface integrin αVβ5 was recognized by a mouse monoclonal anti-human αVβ5 antibody (clone P1F76; sc-13588, Santa Cruz Biotech; 1:200) and stained with AF488-conjugated donkey anti-mouse IgG (ab150105, Abcam) secondary antibody. Another mouse monoclonal anti-human αVβ5 antibody (clone P1F6; 920005, BioLegend; AF647-conjugated) was used to validate the αVβ5 flow cytometry results.
NanoBRET assays
To clone the constructs for the NanoBRET assays (Fig. 4e)39,40, wild-type ITGAV and ITGB5 cDNAs were subcloned from the open reading frame clones (HG11269 for ITGAV and HG10779 for ITGB5, Sino Biological) into the NanoBRET HaloTag and NanoLuc plasmids (N1821, Promega), respectively. Alanine substitution of the ITGAV β-propeller HIP (Fig. 4d,f; mutagenesis primers listed in Supplemental Table 9) was established using a Q5 site-directed mutagenesis kit (E0554S, New England Biolabs). All molecular cloning was performed with NEB 5-α competent E. coli cells (C2987; New England Biolabs). The final plasmids were validated via Sanger sequencing (Eton Bioscience). The NanoBRET HaloTag (ITGAV) and NanoLuc (ITGB5) plasmids were cotransfected into HEK293 cells using FuGENE HD (E2311, Promega). The transfected cells were seeded at 20,000 cells per well in white 96-well tissue culture plates (353296, Falcon) for 24 h and incubated with 100 nM of HaloTag ligand (HaloTag NanoBRET 618 Ligand; generating a 618 nm acceptor signal; G980A, Promega). The wells without HaloTag ligand served as negative controls. When the NanoLuc luciferase substrate was added, the 460 nm donor signal and the 618 nm acceptor signal were measured with a Synergy Neo2 Reader (BioTek).
Transcriptomic analysis
Total RNA from the sgCtrl- and sgITGAV-transduced cell samples was extracted using an RNeasy Mini Kit (74104, QIAGEN). The mRNA library prep was performed by Novogene Inc. and sequenced on a NovaSeq 6000 (Illumina) with ~20 million paired-end 150 bp reads per sample. We then mapped the raw sequence reads to the human GRCh38 genome using STAR v.2.6.1d. Raw counts were quantified using featureCounts v.1.6.4 and then normalized using the trimmed mean of M values method. The relative expression level of each gene was compared using the Bioconductor package ‘edgeR.’ In addition, GSEA (v.4.1.0) was used to evaluate gene pathways affected by sgITGAV.
Purification of ITGAV β-propeller domain
Recombinant protein expression
To clone the pITGAV[31–492 aa] for expressing the recombinant β-propeller domain in E. coli, the full-length human ITGAV open reading frame clone (HG11269, Sino Biological) was PCR amplified (primers AV_BP_F: 5′-GAGAACCTGTACTTCCAATCCATGGAGTTCAACCTAGACGTGGACAG-3′ and AV_BP_R: 5′-GTCGACGGAGCTCGAATTCGGATCCTTAGAGCAGGTTTTATTGTCTTG-3′) and cloned into the pNIC28-Bsa4 vector (26103, Addgene), resulting an ITGAV β-propeller domain (residues Phe31 to Ser492; 50.3 kDa) sequence with an amino-terminal hexahistidine tag (His6-tag). For recombinant expression of the ITGAV β-propeller domain, the pITGAV[31–492 aa] plasmid was first transformed into E. coli (BL21-CodonPlus-RIL; 230240, Agilent Technologies) in the presence of 100 µg ml−1 kanamycin and 50 µg ml−1 chloramphenicol. The transformed E. coli was scaled up to 2 l liquid cultures in Terrific Broth (BP9728-500, ThermoFisher Scientific) at 25 °C until the optical density at 600 nm reached 0.8. Expression of the recombinant β-propeller domain was induced by adding 0.5 mM isopropyl-β-d-thiogalactopyranoside (BP1755-1, ThermoFisher) at 16 °C overnight. The E. coli pellet was collected by centrifugation (8,000g, 4 °C, 5 min) and sonicated (50% amplitude; 5 s bursts interrupted by 5 s pauses for 60 cycles) on ice in the presence of 500 U benzonase (70664, MilliporeSigma) and cOmplete Protease Inhibitor Cocktail (04693159001, Roche). The cell lysate was centrifuged at 10,000g, 4 °C, for 10 min, and the insoluble protein pellet (containing the recombinant protein inclusion body) was harvested for refolding and purification80.
Refolding and purification of the recombinant protein
Briefly, the protein pellet was vortexed to resuspend it in Buffer B (10 mM Tris pH 8.0, 1% Triton X, 0.2 mM phenylmethylsulfonyl fluoride (PMSF)) and Buffer C (10 mM Tris pH 8.0, 0.2 mM PMSF), and the washed protein pellet was collected by centrifugation at 10,000g, 4 °C, for 15 min. This protein pellet was then dissociated in Buffer D (10 mM Tris pH 8.0, 8 M urea, 10 mM DTT, 0.2 mM PMSF) at 4 °C for 2 h and centrifuged at 30,000g, 4 °C, for 30 min. The supernatant was transferred to a prewet dialysis cassette (Slide-A-Lyzer G3, 10K molecular weight cut-off; A52973, ThermoFisher) and submerged in 500 ml of Buffer E (100 mM Tris pH 8.0, 3 M urea, 400 mM l-arginine monohydrochloride, 20 mM reduced l-glutathione, 2 mM oxidized l-glutathione) overnight. Next, the cassette was dialyzed in 2 l of Buffer A (10 mM Tris pH 8.0, 150 mM NaCl) at 4 °C for a total of 24 h (replaced with fresh Buffer A four times). The dialyzed sample (containing the refolded recombinant protein) was then centrifuged at 5,000g, 4 °C for 15 min and filtered through a 0.45 µm PES filter (124-0045, Thermo Scientific Nalgene). The clarified protein sample was further purified by immobilized metal affinity chromatography with a HisTrap HP column (95056-204, Cytiva) followed by anion exchange chromatography with a HiScreen Capto Q column (28926978, Cytiva) using an ÄKTA start protein purification system (GE Healthcare-Cytiva). The purified recombinant ITGAV β-propeller domain protein was checked by SDS polyacrylamide gel electrophoresis with silver staining and stored at −80 °C.
Protein thermal shift assay
The concentration of the purified ITGAV β-propeller domain protein was measured with a DC Protein Assay Kit II (5000112, Bio-Rad). For each thermal shift reaction (50 µl each in 96-well plates), 2.5 µg purified ITGAV β-propeller domain protein was mixed with 40 µM (final concentration) of Cpd_AV2 (or with DMSO as the vehicle control) and Protein Thermal Shift Dye (final concentration preoptimized to 2×; 4462263, ThermoFisher). The protein melt reaction was performed using a QuantStudio 3 real-time PCR system (ThermoFisher) to examine the fluorescence signal (ROX channel) from 25 °C to 99 °C with a ramp rate of 0.1 °C s−1. The protein melting temperature (Tm) and temperature shift (∆Tm) were calculated using JTSA (https://paulsbond.co.uk/jtsa).
Availability of materials
Cas9-expressing cells; ITGAV cDNA; and the CRISPR library for the cell surface proteome (2,973 sgRNAs), integrin family (714 sgRNAs) and ITGAV gene tiling scan (412 sgRNAs) are available upon request. All other biological materials are commercially available.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The RNA sequencing data generated in this study are available via the Gene Expression Omnibus under accession GSE231339. All the data supporting the findings of this study are included in this article and its Supplementary Information. The 3D protein structure (PDB ID: 3IJE) was obtained from the Research Collaboratory for Structural Bioinformatics Protein Data Bank (https://www.rcsb.org). ITGAV expression data for breast, pancreas, brain, colon and lung cancers were obtained from the Gene Expression database of Normal and Tumor tissues (http://gent2.appex.kr/gent2/). Additional data that support the findings of this study are provided in the Supplementary Information.Source data are provided with this paper.
Code availability
The computational code and tool packages used in this study include Genetic Perturbation Platform (BROAD Institute), Bowtie2 (Johns Hopkins University), UCSF Chimera 1.15 (UC San Francisco), Attune NxT v.3.1.2 (ThermoFisher), GSEA v.4.1.0 (UC San Diego and BROAD Institute), FASTQC v.0.11.8, Burrows-Wheeler Aligner v.0.7.17, MACS2 v.2.1.1, SAMtools v.1.10, STAR v.2.6.1d, featureCounts v.1.6.4, edgeR v.4.0.1, deepTools v.3.5.1, IGV 2.14.0 (BROAD Institute), PyMOL v.2.0.4 (Schrödinger, LLC), the PDB 2PQR server76, AutoDockTools77, AutoSite44, Open Babel v.2.4.1 (ref. 78), PyRx v.0.9.7 (ref. 79), AutoDock Vina v.1.1.2 (ref. 45), Raccoon2 (ref. 77), JTSA (https://paulsbond.co.uk/jtsa), Bio-Rad ChemiDoc MP (Bio-Rad), MAGeCK v.0.5.9.2 (ref. 30). IC50 calculations and two-sided Student’s t-tests were performed using Prism 9 (GraphPad). P > 0.05 was considered to indicate a nonsignificant result.
References
von Heijne, G. Membrane-protein topology. Nat. Rev. Mol. Cell Biol. 7, 909–918 (2006).
Thul, P. J. et al. A subcellular map of the human proteome. Science 356, eaal3321 (2017).
Bausch-Fluck, D. et al. The in silico human surfaceome. Proc. Natl Acad. Sci. USA 115, E10988–E10997 (2018).
Bausch-Fluck, D. et al. A mass spectrometric-derived cell surface protein atlas. PLoS ONE 10, e0121314 (2015).
Overington, J. P., Al-Lazikani, B. & Hopkins, A. L. How many drug targets are there? Nat. Rev. Drug Discov. 5, 993–996 (2006).
Hynes, R. O. Integrins: bidirectional, allosteric signaling machines. Cell 110, 673–687 (2002).
Shattil, S. J., Kim, C. & Ginsberg, M. H. The final steps of integrin activation: the end game. Nat. Rev. Mol. Cell Biol. 11, 288–300 (2010).
Arnaout, M. A., Mahalingam, B. & Xiong, J. P. Integrin structure, allostery, and bidirectional signaling. Annu. Rev. Cell Dev. Biol. 21, 381–410 (2005).
Goodman, S. L. & Picard, M. Integrins as therapeutic targets. Trends Pharmacol. Sci. 33, 405–412 (2012).
Desgrosellier, J. S. & Cheresh, D. A. Integrins in cancer: biological implications and therapeutic opportunities. Nat. Rev. Cancer 10, 9–22 (2010).
Slack, R. J., Macdonald, S. J. F., Roper, J. A., Jenkins, R. G. & Hatley, R. J. D. Emerging therapeutic opportunities for integrin inhibitors. Nat. Rev. Drug Discov. 21, 60–78 (2022).
Jinek, M. et al. Structures of Cas9 endonucleases reveal RNA-mediated conformational activation. Science 343, 1247997 (2014).
Hsu, P. D., Lander, E. S. & Zhang, F. Development and applications of CRISPR-Cas9 for genome engineering. Cell 157, 1262–1278 (2014).
Doudna, J. A. & Charpentier, E. Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science 346, 1258096 (2014).
Shalem, O. et al. Genome-scale CRISPR-Cas9 knockout screening in human cells. Science 343, 84–87 (2014).
Wang, T., Wei, J. J., Sabatini, D. M. & Lander, E. S. Genetic screens in human cells using the CRISPR-Cas9 system. Science 343, 80–84 (2014).
Doench, J. G. et al. Optimized sgRNA design to maximize activity and minimize off-target effects of CRISPR-Cas9. Nat. Biotechnol. 34, 184–191 (2016).
Meyers, R. M. et al. Computational correction of copy number effect improves specificity of CRISPR-Cas9 essentiality screens in cancer cells. Nat. Genet. 49, 1779–1784 (2017).
Tsherniak, A. et al. Defining a cancer dependency map. Cell 170, 564–576.e16 (2017).
Shi, J. et al. Discovery of cancer drug targets by CRISPR-Cas9 screening of protein domains. Nat. Biotechnol. 33, 661–667 (2015).
He, W. et al. De novo identification of essential protein domains from CRISPR-Cas9 tiling-sgRNA knockout screens. Nat. Commun. 10, 4541 (2019).
Yang, L. et al. High-resolution characterization of gene function using single-cell CRISPR tiling screen. Nat. Commun. 12, 4063 (2021).
Munoz, D. M. et al. CRISPR screens provide a comprehensive assessment of cancer vulnerabilities but generate false-positive hits for highly amplified genomic regions. Cancer Discov. 6, 900–913 (2016).
Liu, Q. et al. 3-ketodihydrosphingosine reductase maintains ER homeostasis and unfolded protein response in leukemia. Leukemia 36, 100–110 (2022).
Xu, X. et al. ACTR5 controls CDKN2A and tumor progression in an INO80-independent manner. Sci. Adv. 8, eadc8911 (2022).
Sellar, R. S. et al. Degradation of GSPT1 causes TP53-independent cell death in leukemia while sparing normal hematopoietic stem cells. J. Clin. Invest. 132, e153514 (2022).
Uckelmann, H. J. et al. Mutant NPM1 directly regulates oncogenic transcription in acute myeloid leukemia. Cancer Discov. 13, 746–765 (2023).
Li, M. et al. Epigenetic control of translation checkpoint and tumor progression via RUVBL1–EEF1A1 axis. Adv. Sci. 10, e2206584 (2023).
Horlbeck, M. A. et al. Compact and highly active next-generation libraries for CRISPR-mediated gene repression and activation. eLife 5, e19760 (2016).
Li, W. et al. MAGeCK enables robust identification of essential genes from genome-scale CRISPR/Cas9 knockout screens. Genome Biol. 15, 554 (2014).
Tang, Z. et al. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 45, W98–W102 (2017).
Wiesner, S., Legate, K. R. & Fassler, R. Integrin-actin interactions. Cell. Mol. Life Sci. 62, 1081–1099 (2005).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005).
Schoonenberg, V. A. C. et al. CRISPRO: identification of functional protein coding sequences based on genome editing dense mutagenesis. Genome Biol. 19, 169 (2018).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Xiong, J. P. et al. Crystal structure of the extracellular segment of integrin αVβ3. Science 294, 339–345 (2001).
Gupta, V. et al. Role of the β-subunit arginine/lysine finger in integrin heterodimer formation and function. J. Immunol. 180, 1713–1718 (2008).
Takagi, J., DeBottis, D. P., Erickson, H. P. & Springer, T. A. The role of the specificity-determining loop of the integrin β subunit I-like domain in autonomous expression, association with the α subunit, and ligand binding. Biochemistry 41, 4339–4347 (2002).
Machleidt, T. et al. NanoBRET – a novel BRET platform for the analysis of protein-protein interactions. ACS Chem. Biol. 10, 1797–1804 (2015).
Dale, N. C., Johnstone, E. K. M., White, C. W. & Pfleger, K. D. G. NanoBRET: the bright future of proximity-based assays. Front. Bioeng. Biotechnol. 7, 56 (2019).
Dong, X. et al. Force interacts with macromolecular structure in activation of TGF-β. Nature 542, 55–59 (2017).
Xiong, J. P. et al. Crystal structure of the extracellular segment of integrin αVβ3 in complex with an Arg-Gly-Asp ligand. Science 296, 151–155 (2002).
Xiong, J. P. et al. Crystal structure of the complete integrin αVβ3 ectodomain plus an α/β transmembrane fragment. J. Cell Biol. 186, 589–600 (2009).
Ravindranath, P. A. & Sanner, M. F. AutoSite: an automated approach for pseudo-ligands prediction-from ligand-binding sites identification to predicting key ligand atoms. Bioinformatics 32, 3142–3149 (2016).
Trott, O. & Olson, A. J. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 31, 455–461 (2010).
Oliveira-Ferrer, L. et al. Cilengitide induces cellular detachment and apoptosis in endothelial and glioma cells mediated by inhibition of FAK/src/AKT pathway. J. Exp. Clin. Cancer Res. 27, 86 (2008).
Burke, P. A. et al. Cilengitide targeting of αvβ3 integrin receptor synergizes with radioimmunotherapy to increase efficacy and apoptosis in breast cancer xenografts. Cancer Res. 62, 4263–4272 (2002).
Cheuk, I. W. et al. ITGAV targeting as a therapeutic approach for treatment of metastatic breast cancer. Am. J. Cancer Res 10, 211–223 (2020).
Bader, B. L., Rayburn, H., Crowley, D. & Hynes, R. O. Extensive vasculogenesis, angiogenesis, and organogenesis precede lethality in mice lacking all αv integrins. Cell 95, 507–519 (1998).
Friedlander, M. et al. Involvement of integrins alpha v beta 3 and alpha v beta 5 in ocular neovascular diseases. Proc. Natl Acad. Sci. USA 93, 9764–9769 (1996).
Asano, Y. et al. Involvement of αvβ5 integrin-mediated activation of latent transforming growth factor β1 in autocrine transforming growth factor β signaling in systemic sclerosis fibroblasts. Arthritis Rheumatol. 52, 2897–2905 (2005).
Sheppard, D. Integrin-mediated activation of latent transforming growth factor β. Cancer Metastasis Rev. 24, 395–402 (2005).
Uhm, J. H., Dooley, N. P., Kyritsis, A. P., Rao, J. S. & Gladson, C. L. Vitronectin, a glioma-derived extracellular matrix protein, protects tumor cells from apoptotic death. Clin. Cancer Res. 5, 1587–1594 (1999).
Lane, D., Goncharenko-Khaider, N., Rancourt, C. & Piche, A. Ovarian cancer ascites protects from TRAIL-induced cell death through αvβ5 integrin-mediated focal adhesion kinase and Akt activation. Oncogene 29, 3519–3531 (2010).
Zheng, Y. et al. Role of myeloma-derived MIF in myeloma cell adhesion to bone marrow and chemotherapy response. J. Natl Cancer Inst. 108, djw131 (2016).
Lee, Y. S. et al. Inhibition of skin carcinogenesis by suppression of NF-κB dependent ITGAV and TIMP-1 expression in IL-32γ overexpressed condition. J. Exp. Clin. Cancer Res. 37, 293 (2018).
Kang, C. L. et al. LncRNA AY promotes hepatocellular carcinoma metastasis by stimulating ITGAV transcription. Theranostics 9, 4421–4436 (2019).
Linhares, M. M. et al. Genetic and immunohistochemical expression of integrins ITGAV, ITGA6, and ITGA3 as prognostic factor for colorectal cancer: models for global and disease-free survival. PLoS ONE 10, e0144333 (2015).
Azimian-Zavareh, V. et al. Wnt5A modulates integrin expression in a receptor-dependent manner in ovarian cancer cells. Sci. Rep. 11, 5885 (2021).
Loeser, H. et al. Integrin alpha V (ITGAV) expression in esophageal adenocarcinoma is associated with shortened overall-survival. Sci. Rep. 10, 18411 (2020).
Zemskova, M. Y. et al. Integrin alpha V in urine: a novel noninvasive marker for prostate cancer detection. Front Oncol. 10, 610647 (2020).
Kemper, M. et al. Integrin alpha-V is an important driver in pancreatic adenocarcinoma progression. J. Exp. Clin. Cancer Res. 40, 214 (2021).
Zhu, Z. et al. Zika virus targets glioblastoma stem cells through a SOX2-integrin αvβ5 axis. Cell Stem Cell 26, 187–204.e10 (2020).
Hatley, R. J. D. et al. An αv-RGD integrin inhibitor toolbox: drug discovery insight, challenges and opportunities. Angew. Chem. Int. Ed. 57, 3298–3321 (2018).
Elez, E. et al. Abituzumab combined with cetuximab plus irinotecan versus cetuximab plus irinotecan alone for patients with KRAS wild-type metastatic colorectal cancer: the randomised phase I/II POSEIDON trial. Ann. Oncol. 26, 132–140 (2015).
Stupp, R. et al. Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071-22072 study): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 15, 1100–1108 (2014).
Reynolds, A. R. et al. Stimulation of tumor growth and angiogenesis by low concentrations of RGD-mimetic integrin inhibitors. Nat. Med. 15, 392–400 (2009).
Hariharan, S. et al. Assessment of the biological and pharmacological effects of the ανβ3 and ανβ5 integrin receptor antagonist, cilengitide (EMD121974), in patients with advanced solid tumors. Ann. Oncol. 18, 1400–1407 (2007).
Kemperman, H., Wijnands, Y. M. & Roos, E. alphaV integrins on HT-29 colon carcinoma cells: adhesion to fibronectin is mediated solely by small amounts of alphaVbeta6, and alphaVbeta5 is codistributed with actin fibers. Exp. Cell. Res. 234, 156–164 (1997).
Marsico, G., Russo, L., Quondamatteo, F. & Pandit, A. Glycosylation and integrin regulation in cancer. Trends Cancer 4, 537–552 (2018).
Filippakopoulos, P. et al. Selective inhibition of BET bromodomains. Nature 468, 1067–1073 (2010).
Elkins, J. M., Santaguida, S., Musacchio, A. & Knapp, S. Crystal structure of human aurora B in complex with INCENP and VX-680. J. Med. Chem. 55, 7841–7848 (2012).
Brown, N. R. et al. CDK1 structures reveal conserved and unique features of the essential cell cycle CDK. Nat. Commun. 6, 6769 (2015).
Zhu, J. Y. et al. Structural basis of Wee kinases functionality and inactivation by diverse small molecule inhibitors. J. Med. Chem. 60, 7863–7875 (2017).
Pettersen, E. F. et al. UCSF Chimera–a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Dolinsky, T. J. et al. PDB 2PQR: expanding and upgrading automated preparation of biomolecular structures for molecular simulations. Nucleic Acids Res. 35, W522–W525 (2007).
Forli, S. et al. Computational protein-ligand docking and virtual drug screening with the AutoDock suite. Nat. Protoc. 11, 905–919 (2016).
O’Boyle, N. M. et al. Open Babel: an open chemical toolbox. J. Cheminform. 3, 33 (2011).
Dallakyan, S. & Olson, A. J. Small-molecule library screening by docking with PyRx. Methods Mol. Biol. 1263, 243–250 (2015).
Machuca, M. A. & Roujeinikova, A. Method for efficient refolding and purification of chemoreceptor ligand binding domain. J. Vis. Exp. https://doi.org/10.3791/57092 (2017).
Acknowledgements
We thank the NCI/DTP (https://dtp.cancer.gov) for providing the compound library. This work was supported by the American Society of Hematology (C.-W.C.); Alex’s Lemonade Stand Foundation 18-11849 (C.-W.C.); Stand Up To Cancer & Cancer Research UK RT617 (C.-W.C.); and National Institutes of Health grants CA197489, CA233691, CA236626, CA243124, CA278050 (C.-W.C.), CA274649 (N.M.M.), CA233922 (S.T.R.), CA255250 and CA258778 (M.F.). The structural computational studies were supported by National Institutes of Health grant GM117923 (N.V.), and the sequencing, mass spectrometry and structural computational studies were supported by National Institutes of Health P30 award CA033572 (City of Hope Cancer Center). These funders were not involved in study design, data collection or analysis, the decision to publish or the preparation of the paper.
Author information
Authors and Affiliations
Contributions
N.M.M., A.K.N.C., K.M., W.-H.C., S.P.P., M.L., Q.L., X.X., R.C., P.S., L.Z., Z.E., B.C., D.K. and M.F. performed the experiments. N.M.M., A.K.N.C, L.Y., Y.W., P.P., R.C.R. and C.-W.C. analyzed the data. N.M.M. and A.K.N.C. performed in silico molecular docking. E.M., N.M. and N.V. performed and analyzed the molecular dynamics simulations. S.T.R., J.C., M.A.L., J.S., V.N., R.C.R., M.F. and C.-W.C. provided conceptual input. N.M.M. and C.-W.C wrote the paper. C.-W.C. conceived and supervised the study.
Corresponding author
Ethics declarations
Competing interests
J.C. is a scientific advisory board member of Race Oncology. The other authors declare no competing interests.
Peer review
Peer review information
Nature Structural & Molecular Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available. Primary Handling Editor: Dimitris Typas, in collaboration with the Nature Structural & Molecular Biology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 CRISPR genetic screen libraries used in this study.
(a) Map of the ipUSEPR vector expressing a sgRNA together with a puromycin-resistant gene (PuroR) and a TagRFP fluorescent protein. Primers for Sanger (hU6-F_seq) and Illumina (DCF01 and DCR03) sequencing are listed. (b–d) Design and distribution of individual sgRNA frequencies RPMR (reads per million reads) in the CRISPR libraries targeting (B) cell surface proteome genes (n = 2,973 sgRNAs), (C) integrin family genes (n = 714 sgRNAs), and (D) coding regions of ITGAV (n = 412 sgRNAs). (B) 90.1%, (C) 97.7%, and (D) 96.3% of sgRNA in these libraries passed the QC by exhibiting RPMR ≥ 10. Data are represented as median ± interquartile range.
Extended Data Fig. 2 Analyses of the surface proteome CRISPR library screens.
(a) Combined gene ranking of the cell surface proteome CRISPR screens was calculated by the MAGeCK algorithm. The ranking of ITGAV (red), positive controls (yellow; target common essential genes), negative controls (green; target non-essential sequences), and total library (grey) are indicated. The pink box highlights the leading-edge essential genes with a combined Log2FC below -1.0. (b) Distribution of the positive (n = 12 genes) and negative (n = 5 genes) controls in the screen. Data are represented as median ± interquartile range. P value was calculated by two-sided Student’s t-test. (c) Three surface protein genes (ITGAV, ATP6AP2, and TFRC) were identified as the leading-edge essential genes. (d) Correlation of the CERES scores (computational method to estimate gene-dependency levels from CRISPR-Cas9 essentiality screens) and gene expression of ITGAV (left panel), ATP6AP2 (middle panel), and TFRC (right panel)(source: https://depmap.org/portal/; BROAD Institute). The cancer cell dependency on ITGAV is correlated with its expression. (e) Top ten candidate hits and (f) an overlap plot of the surface proteome CRISPR screens in five cell models. Red (ITGAV and ATP6AP2) indicates the common essential surface proteins in all screened cell types. Other colors (blue, orange, green, cyan, and pink) highlight the cell type-specific candidate genes.
Extended Data Fig. 3 Schematic outline of the flow cytometric analysis.
(a) RFP growth competition assay (used in Figs. 1e, 2d, 3e): The ipUSEPR vector expresses a sgRNA together with a puromycin-resistant gene (PuroR) and a TagRFP fluorescent protein. The RFP fluorescent signal of live (DAPI–) singlet cells was detected by an Attune NxT flow cytometer with an HTS autosampler. The sgRNA targeting a functionally important gene will result in a reduced RFP+ population in the culture. (b) Gating strategy for detecting the cell surface αVβ5 expression (used in Fig. 5e, f).
Extended Data Fig. 4 The effect of sgITGAV can be reversed by the exogenous ITGAV cDNA.
(a) Schematic outline of the ITGAV gene coding region. The recognition sites of sgITGAV#2 and sgITGAV#3 span across the exon-intron junctions. These sgITGAVs only target the endogenous ITGAV coding sequence (with introns) but cannot recognize the ITGAV cDNA sequence (w/o introns), thus allowing the reconstitution of ITGAV through cDNA transduction. (b) Transduction of exogenous ITGAV cDNA in MDA231 cells significantly reversed the impact of sgITGAV on cell survival (n = 3 for each group). Data are represented as mean ± s.e.m. P values were calculated by two-sided Student’s t-test.
Extended Data Fig. 5 Correlation of the CERES scores between ITGAV and other genes.
CERES score is a computational method to estimate gene-dependency levels from CRISPR-Cas9 essentiality screens. The CERES scores of ITGAV (x-axis) and (a) Rho small GTPase genes RAC1, CDC42, and RHOA (y-axes; left, middle, and right respectively) and (b) ITGB1/3/5/6/8 (y-axes; top-left, top-middle, top-right, bottom-middle, bottom-right, respectively) in 769 cell models (dots) were obtained from the DepMap CRISPR screen consortium database (source: https://depmap.org/portal/; BROAD Institute). A higher Pearson coefficient (r) of the CERES scores between two genes indicates a higher likelihood the two genes are co-regulated in the tested cell models. The gene rank number is based on the Pearson coefficient (r) of the CERES scores between ITGAV and a total of 17,709 genes tested in the genome-wide CRISPR library screens.
Extended Data Fig. 6 Effect of alanine substitution of non-HIP aromatic residues on the NanoBRET assay.
(a) The location of four aromatic residues (W234, F507, W790, and F938) outside of HIP were highlighted. (b) Alanine substitution of these non-HIP residues (purple; n = 3 for each group) exhibits minimal impact on the NanoBRET signal compared to the wild-type ITGAV (gray; n = 3). Data are represented as mean ± s.e.m. P values were calculated by two-sided Student’s t-test.
Extended Data Fig. 7 Information of the candidate ITGAV targeting compounds.
The NCI/DTP NSC identifier, the predicted binding free energy (ΔG°) to ITGAV’s β-propeller HIP, and the chemical structure of the top 9 candidate compounds are indicated. The identity (up-right; within one ppm of theoretical value) of Cpd_AV2 was validated by an Orbitrap Fusion Tribrid Mass Spectrometer (Thermo Scientific) at the City of Hope Integrated Mass Spectrometry Shared Resource.
Extended Data Fig. 8 Modeling of ITGAV/ITGB5 interaction.
(a) 3D structure of the extracellular domain of ITGB5 was modeled by AlphaFold2 (cyan) and overlaid with the ITGB3 portion of integrin αVβ3 structure resolved by Xiong et al. (PDB ID: 3IJE, chain B; blue). Overall, we observed high concordance of the 3D structures between ITGB3 and ITGB5, including the highly conserved basic amino acid (ITGB3’s R287 or ITGB5’s K287) in the loop motif of the βA domain highlighted in (b). (c) Modeling of ITGAV/ITGB5 interaction using the AlphaFold2 predicted ITGB5 structure (cyan) and the ITGAV portion of integrin αVβ3 structure (PDB ID: 3IJE, chain A; red). (d and e) Molecular dynamics simulation using GROMACS 2022 with CHARMM36m force field indicates (d) a close contact between ITGB5’s K287 and ITGAV’s β-propeller HIP pocket (purple box), and (e) the occupancy of Cpd_AV2 (yellow) into ITGAV’s HIP pocket disengaged the side chain of ITGB5’s K287 from stably interacting with ITGAV. (f) Substitution of ITGB5’s K287 with an alanine (K287A) significantly attenuated the ITGAV/ITGB5 NanoBRET signal, highlighting an essential role of this basic residue in integrin αVβ5 assembly (n = 3 for each group). Data are represented as mean ± s.e.m. P value was calculated by two-sided Student’s t-test.
Extended Data Fig. 9 Potential impact of Cpd_AV2 on additional ITGAV integrin pairs.
(a) Sequence alignment of ITGAV heterodimer partners ITGB1/3/5/6/8 at the loop motif of their βA domain. The highly conserved basic amino acid (K/R287) encapsulated in ITGAV’s β-propeller is labeled. (b) Cpd_AV2 treatment attenuates the integrin αVβ6-mediated adhesion to fibronectin (Fn) in HT-29 colorectal carcinoma cells (n = 3 for each group).
Extended Data Fig. 10 CRISPR-TICA evaluation of well-defined drug-targeting pockets.
The smoothened CRISPR tiling data (left panel; blue lines) of (a) BRD4, (b) AURKB, (c) CDK1, and (d) WEE1 were obtained from Munoz et al. On the right panels, the green boxes highlight the CRISPR-TICA region of interest based on the CRISPR sensitivity. The yellow arrows indicate the previously reported inhibitors for these proteins.
Supplementary information
Supplementary Information
Supplementary Figs. 1–4.
Supplementary Tables
Supplementary Tables.
Supplementary Data 1
Supplementary Data 1.
Supplementary Data 2
Supplementary Data 2.
Supplementary Data 3
Supplementary Data 3.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2.
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 9
Statistical source data.
Source Data Extended Data Fig. 10
Statistical source data.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mattson, N.M., Chan, A.K.N., Miyashita, K. et al. A novel class of inhibitors that disrupts the stability of integrin heterodimers identified by CRISPR-tiling-instructed genetic screens. Nat Struct Mol Biol 31, 465–475 (2024). https://doi.org/10.1038/s41594-024-01211-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41594-024-01211-y