Abstract
Microglia are resident immune cells of the central nervous system and play key roles in brain homeostasis. During anesthesia, microglia increase their dynamic process surveillance and interact more closely with neurons. However, the functional significance of microglial process dynamics and neuronal interaction under anesthesia is largely unknown. Using in vivo two-photon imaging in mice, we show that microglia enhance neuronal activity after the cessation of isoflurane anesthesia. Hyperactive neuron somata are contacted directly by microglial processes, which specifically colocalize with GABAergic boutons. Electron-microscopy-based synaptic reconstruction after two-photon imaging reveals that, during anesthesia, microglial processes enter into the synaptic cleft to shield GABAergic inputs. Microglial ablation or loss of microglial β2-adrenergic receptors prevents post-anesthesia neuronal hyperactivity. Our study demonstrates a previously unappreciated function of microglial process dynamics, which enable microglia to transiently boost post-anesthesia neuronal activity by physically shielding inhibitory inputs.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The raw data that support the findings of this study are available from the corresponding author upon request. Source data are provided with this paper.
Code availability
All ImageJ macro scripts for analysis will be made available on request (only basic ImageJ functions such as batch processing were performed).
References
Davalos, D. et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci. 8, 752–758 (2005).
Kettenmann, H., Hanisch, U. K., Noda, M. & Verkhratsky, A. Physiology of microglia. Physiol. Rev. 91, 461–553 (2011).
Nimmerjahn, A., Kirchhoff, F. & Helmchen, F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308, 1314–1318 (2005).
Paolicelli, R. C. et al. Synaptic pruning by microglia is necessary for normal brain development. Science 333, 1456–1458 (2011).
Schafer, D. P. et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 74, 691–705 (2012).
Wake, H., Moorhouse, A. J., Jinno, S., Kohsaka, S. & Nabekura, J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J. Neurosci. 29, 3974–3980 (2009).
Tremblay, M.-È., Lowery, R. L. & Majewska, A. K. Microglial interactions with synapses are modulated by visual experience. PLoS Biol. 8, e1000527 (2010).
Akiyoshi, R. et al. Microglia enhance synapse activity to promote local network synchronization.eNeuro 5, ENEURO.0088-18.2018 (2018).
Eyo, U. B. et al. Neuronal hyperactivity recruits microglial processes via neuronal NMDA receptors and microglial P2Y12 receptors after status epilepticus. J. Neurosci. 34, 10528–10540 (2014).
Wu, W. et al. Microglial depletion aggravates the severity of acute and chronic seizures in mice. Brain Behav Immun 89, 245–255 (2020).
Badimon, A. et al. Negative feedback control of neuronal activity by microglia. Nature 586, 417–423 (2020).
Cserep, C. et al. Microglia monitor and protect neuronal function through specialized somatic purinergic junctions. Science 367, 528–537 (2020).
Colon, E. et al. Anesthesia, brain changes, and behavior: Insights from neural systems biology. Prog. Neurobiol. 153, 121–160 (2017).
Ou, M. et al. The general anesthetic isoflurane bilaterally modulates neuronal excitability. iScience 23, 100760 (2020).
Liu, Y. U. et al. Neuronal network activity controls microglial process surveillance in awake mice via norepinephrine signaling. Nat. Neurosci. 22, 1771–1781 (2019).
Stowell, R. D. et al. Noradrenergic signaling in the wakeful state inhibits microglial surveillance and synaptic plasticity in the mouse visual cortex. Nat. Neurosci. 22, 1782–1792 (2019).
Wan, Y. et al. Microglial displacement of GABAergic synapses is a protective event during complex febrile seizures. Cell Rep. 33, 108346 (2020).
Favuzzi, E. et al. GABA-receptive microglia selectively sculpt developing inhibitory circuits. Cell 184, 4048–4063 e4032 (2021).
Logiacco, F. et al. Microglia sense neuronal activity via GABA in the early postnatal hippocampus. Cell Rep. 37, 110128 (2021).
Bishop, D. et al. Near-infrared branding efficiently correlates light and electron microscopy. Nat. Methods 8, 568–570 (2011).
Feng, J. et al. A genetically encoded fluorescent sensor for rapid and specific in vivo detection of norepinephrine. Neuron 102, 745–761 e748 (2019).
Wilton, D. K., Dissing-Olesen, L. & Stevens, B. Neuron-Glia signaling in synapse elimination. Annu. Rev. Neurosci. 42, 107–127 (2019).
Miyamoto, A. et al. Microglia contact induces synapse formation in developing somatosensory cortex. Nat. Commun. 7, 12540 (2016).
Parkhurst, C. N. et al. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell 155, 1596–1609 (2013).
Blinzinger, K. & Kreutzberg, G. Displacement of synaptic terminals from regenerating motoneurons by microglial cells. Z. Zellforsch. Mikrosk. Anat. 85, 145–157 (1968).
Trapp, B. D. et al. Evidence for synaptic stripping by cortical microglia. Glia 55, 360–368 (2007).
Chen, Z. et al. Microglial displacement of inhibitory synapses provides neuroprotection in the adult brain. Nat. Commun. 5, 4486 (2014).
Hattori, R., Kuchibhotla, K. V., Froemke, R. C. & Komiyama, T. Functions and dysfunctions of neocortical inhibitory neuron subtypes. Nat. Neurosci. 20, 1199–1208 (2017).
Zhang, Y. et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. 34, 11929–11947 (2014).
Ravi, B. et al. Association of duration of surgery with postoperative delirium among patients receiving hip fracture repair. JAMA Netw. Open 2, e190111 (2019).
Wu, X. et al. The inhalation anesthetic isoflurane increases levels of proinflammatory TNF-alpha, IL-6, and IL-1beta. Neurobiol. Aging 33, 1364–1378 (2012).
Lodder, C. et al. CSF1R inhibition rescues tau pathology and neurodegeneration in an A/T/N model with combined AD pathologies, while preserving plaque associated microglia. Acta Neuropathol. Commun. 9, 108 (2021).
Zhan, L. et al. A MAC2-positive progenitor-like microglial population is resistant to CSF1R inhibition in adult mouse brain. eLife 9, e51796 (2020).
Liu, Y. et al. Touch and tactile neuropathic pain sensitivity are set by corticospinal projections. Nature 561, 547–550 (2018).
Kim, W., Kim, S. K. & Nabekura, J. Functional and structural plasticity in the primary somatosensory cortex associated with chronic pain. J. Neurochem. 141, 499–506 (2017).
Koponen, H., Partanen, J., Paakkonen, A., Mattila, E. & Riekkinen, P. J. EEG spectral analysis in delirium. J. Neurol. Neurosurg. Psychiatry 52, 980–985 (1989).
Kalen, P., Rosegren, E., Lindvall, O. & Bjorklund, A. Hippocampal noradrenaline and serotonin release over 24 hours as measured by the dialysis technique in freely moving rats: correlation to behavioural activity state, effect of handling and Tail-pinch. Eur. J. Neurosci. 1, 181–188 (1989).
Bellesi, M. et al. Sleep loss promotes astrocytic phagocytosis and microglial activation in mouse cerebral cortex. J. Neurosci. 37, 5263–5273 (2017).
Choudhury, M. E. et al. Phagocytic elimination of synapses by microglia during sleep. Glia 68, 44–59 (2020).
Liu, H. et al. Microglia modulate stable wakefulness via the thalamic reticular nucleus in mice. Nat. Commun. 12, 4646 (2021).
Gyoneva, S. & Traynelis, S. F. Norepinephrine modulates the motility of resting and activated microglia via different adrenergic receptors. J. Biol. Chem. 288, 15291–15302 (2013).
Brown, E. N., Lydic, R. & Schiff, N. D. General anesthesia, sleep, and coma. N. Engl. J. Med. 363, 2638–2650 (2010).
Umpierre, A. D. et al. Microglial calcium signaling is attuned to neuronal activity in awake mice. eLife 9, e56502 (2020).
Thevenaz, P., Ruttimann, U. E. & Unser, M. A pyramid approach to subpixel registration based on intensity. IEEE Trans. Image Process. 7, 27–41 (1998).
Yi, M. H. et al. Optogenetic activation of spinal microglia triggers chronic pain in mice. PLoS Biol. 19, e3001154 (2021).
Gu, N. et al. Spinal microglia contribute to sustained inflammatory pain via amplifying neuronal activity. Mol. Brain 15, 86 (2022).
Deerinck, T. J., Bushong, E. A., Thor, A. & Ellisman, M. H. NCMIR methods for 3D EM: A new protocol for preparation of biological specimens for serial block face scanning electron microscopy. National Center for Microscopy and Imaging Research, University of California San Diego, La Jolla, CA (2010).
Willingham, M. C. & Rutherford, A. V. The use of osmium-thiocarbohydrazide-osmium (OTO) and ferrocyanide-reduced osmium methods to enhance membrane contrast and preservation in cultured cells. J. Histochem. Cytochem. 32, 455–460 (1984).
Fiala, J. C. Reconstruct: a free editor for serial section microscopy. J. Microsc. 218, 52–61 (2005).
Garaschuk, O. & Verkhratsky, A. Physiology of microglia. Methods Mol. Biol. 2034, 27–40 (2019).
Gray, E. G. Axo-somatic and axo-dendritic synapses of the cerebral cortex: an electron microscope study. J. Anat. 93, 420–433 (1959).
Kremen, V. et al. Behavioral state classification in epileptic brain using intracranial electrophysiology. J. Neural Eng. 14, 026001 (2017).
Acknowledgements
We thank Y. Li (Peking University) for critical input on NE detection in vivo. We thank D. B. Bosco for proofreading the manuscript and members of the Wu Laboratory for insightful discussions. We thank the Mayo Clinic Microscopy and Cell Analysis Core facility for experimental and technical support. This work is supported by the National Institutes of Health (Grant Nos. R01NS088627, R01NS112144 and R35NS132326 to L.-J.W.). Y.Y. was partially supported by National Natural Science Foundation of China (Grant No. 82001137).
Author information
Authors and Affiliations
Contributions
K.H., Y.Y. and L.-J.W. designed the study. K.H., A.D.U. and L.-J.W. wrote the manuscript. K.H., T.C. and Y.Y. performed animal surgery, image collection and data analyses. K.H. and F.Q. performed immunofluorescence staining experiments and electron microscope experiments. K.H., F.Q. and T.X. performed 3D image reconstruction. S.Z. and J.Z. performed qPCR experiments. K.H., Y.Y. and M.-H.Y. performed behavioral tests and analysis. K.H., Y.Y., Y.L., V.K. and G.A.W. analyzed EEG data. Y.U.L. and H.D. assisted with some experimental design and manuscript revision. Funding was obtained and the project was supervised by L.-J.W.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Neuroscience thanks Ádám Dénes, Ania Majewska and Marie-Ève Tremblay for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
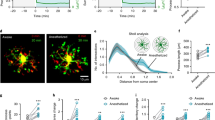
Extended Data Fig. 1 Increased neuronal calcium activity and microglial territory changes during emergence from anesthesia in both male and female mice.
a–c, Neuronal Ca2+ signal amplitude (a), time active (b) and signal area (c) across experimental time points for male mice and female mice (amplitude: Awake versus Isoflurane, p = 0.7343; Awake versus Emergence 15 min, p = 0.1393; Awake versus Emergence 30 min, p > 0.9999; Awake versus Emergence 45 min, p > 0.9999; Awake versus Emergence 60 min, p = 0.9486. time active: Awake versus Isoflurane, p = 0.9915; Awake versus Emergence 15 min, p = 0.9187; Awake versus Emergence 30 min, p = 0.9988; Awake versus Emergence 45 min, p = 0.9432; Awake versus Emergence 60 min, p = 0.4240. signal area: Awake versus Isoflurane, p > 0.9999; Awake versus Emergence 15 min, p > 0.9999; Awake versus Emergence 30 min, p = 0.9563; Awake versus Emergence 45 min, p = 0.4973; Awake versus Emergence 60 min, p = 0.1640). Solid lines represent the mean ± SEM from n = 5 mice, while dashed lines indicate individual animals. d, Representative images of microglial morphology in female mouse during each experimental phase (Scale bar: 10 μm). e, Time-course changes in microglial territory (territory: Awake versus Isoflurane 15 min, p > 0.9999; Awake versus Isoflurane 30 min, p = 0.4066; Awake versus Emergence 15 min, p = 0.9920; Awake versus Emergence 30 min, p = 0.5578; Awake versus Emergence 45 min, p > 0.9999; Awake versus Emergence 60 min, p > 0.9999). Solid lines represent the mean ± SEM from male: n = 6 mice, female: n = 5 mice). Two-way ANOVA followed by Sidak post-hoc test; n.s., not significant. All experiments were repeated multiple times using different mice independently with similar results obtained.
Extended Data Fig. 2 Control chow and PLX chow feeding in neuronal activity during emergence from anesthesia.
a, Experimental timeline of control chow feeding and two-photon imaging of neuronal Ca2+ activity. Related to main Fig. 2. b–d, Neuronal Ca2+ signal amplitude (b), time active (c) and signal area (d) across experimental time points for control chow 3-weeks group and 6-weeks group(amplitude: Awake versus Isoflurane, p = 0.9676; Awake versus Emergence 15 min, p = 0.8172; Awake versus Emergence 30 min, p = 0.9713; Awake versus Emergence 45 min, p = 0.9911; Awake versus Emergence 60 min, p = 0.9530. time active: Awake versus Isoflurane, p > 0.9999; Awake versus Emergence 15 min, p = 0.9733; Awake versus Emergence 30 min, p = 0.9897; Awake versus Emergence 45 min, p = 0.9697; Awake versus Emergence 60 min, p = 0.9995. signal area: Awake versus Isoflurane, p > 0.9999; Awake versus Emergence 15 min, p > 0.9999; Awake versus Emergence 30 min, p = 0.9754; Awake versus Emergence 45 min, p = 0.9992; Awake versus Emergence 60 min, p > 0.9999). Solid lines represent the mean ± SEM from n = 4 mice. e–g, Neuronal Ca2+ signal amplitude (e), time active (f) and signal area (g) across experimental time points for before and after microglia ablation at each time point (amplitude: Awake versus Isoflurane, p = 0.9959; Awake versus Emergence 15 min, p < 0.0001; Awake versus Emergence 30 min, p < 0.0001; Awake versus Emergence 45 min, p < 0.0001; Awake versus Emergence 60 min, p = 0.0413. time active: Awake versus Isoflurane, p > 0.9999; Awake versus Emergence 15 min, p = 0.0069; Awake versus Emergence 30 min, p = 0.0009; Awake versus Emergence 45 min, p = 0.0601; Awake versus Emergence 60 min, p = 0.8613. signal area: Awake versus Isoflurane, p > 0.9999; Awake versus Emergence 15 min, p = 0.1259; Awake versus Emergence 30 min, p = 0.0013; Awake versus Emergence 45 min, p = 0.0239; Awake versus Emergence 60 min, p = 0.0657). Solid lines represent the mean ± SEM from n = 10 mice. h–j, Absolute value of neuronal Ca2+ signal amplitude (h), time active (i) and signal area (j) (amplitude: Awake versus Isoflurane, p = 0.9998; Awake versus Emergence 15 min, p = 0.7250; Awake versus Emergence 30 min, p = 0.7176; Awake versus Emergence 45 min, p = 0.9757; Awake versus Emergence 60 min, p > 0.9999. time active: Awake versus Isoflurane, p > 0.9999; Awake versus Emergence 15 min, p = 0.4355; Awake versus Emergence 30 min, p = 0.5141; Awake versus Emergence 45 min, p > 0.9999; Awake versus Emergence 60 min, p = 0.8270. signal area: Awake versus Isoflurane, p > 0.9999; Awake versus Emergence 15 min, p = 0.9626; Awake versus Emergence 30 min, p = 0.8873; Awake versus Emergence 45 min, p > 0.9999; Awake versus Emergence 60 min, p = 0.9442). Solid lines represent the mean ± SEM from n = 10 mice. Two-way ANOVA followed by Sidak post-hoc test (b–j); n.s., not significant; *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001.
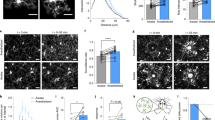
Extended Data Fig. 3 Increased contacts between microglia and neuronal somata or dendrites in response to general anesthesia.
a, Two-photon imaging of microglia (Cx3cr1GFP/+, green) and neurons co-labelled with GCaMP6s (green) and tdTomato (red). Representative time series images of interaction between microglial bulbous endings (green) and neuronal soma (red) during anesthesia. White arrowheads indicate contact sites. Scale bar: 10 μm. b, Graph shows that number of microglia-neuronal soma contact sites before (Awake), during (Isoflurane) and after anesthesia (Emergence) was significantly increased during anesthesia (Awake versus Isoflurane, p = 0.0030; Awake versus Emergence, p = 0.0199; Isoflurane versus Recovery, p = 0.0640). Solid lines represent the mean ± SEM from n = 5 imaging field from 5 mice. c, Duration of microglial interaction (Awake versus Isoflurane, p = 0.0020; Awake versus Emergence, p = 0.0079; Isoflurane versus Recovery, p = 0.7197). n = 5 imaging field from 5 mice. d, Representative time series images of interaction between microglial bulbous endings (green) and neuronal dendrites (red) before, during, and after anesthesia. Microglia-neuronal dendrite interaction was increased during anesthesia (indicated by white arrowheads). Scale bar: 5 μm. e, Graph shows number of microglia-neuronal dendrite contact sites per 100 μm length of dendrite (Awake versus Isoflurane, p = 0.0016; Awake versus Emergence, p = 0.9572; Isoflurane versus Recovery, p = 0.0041). Solid lines represent the mean ± SEM from n = 6 imaging fields from 3 mice. f, Graph shows duration of observed microglia-dendrite interactions before, during, and after anesthesia (Awake versus Isoflurane, p = 0.2029; Awake versus Emergence, p = 0.0174; Isoflurane versus Recovery, p = 0.3960). n = 6 imaging fields from 3 mice. g, Representative velocity changes in microglial bulbous ending in contact with neurons. h-i, Time-course of velocity changes in microglial bulbous endings (h) and normal processes (i, excluding bulbous endings) during awake, anesthesia, and emergence (bulbous endings: Awake versus Isoflurane, p = 0.8423, Awake versus Emergence 15 min, p = 0.7476; Awake versus Emergence 30 min, p = 0.5805; Awake versus Emergence 45 min, p = 0.1302; Awake versus Emergence 60 min, p = 0.0237. normal processes: Awake versus Isoflurane, p = 0.0447; Awake versus Emergence 15 min, p = 0.9919; Awake versus Emergence 30 min, p = 0.8353; Awake versus Emergence 45 min, p = 0.5313; Awake versus Emergence 60 min, p = 0.2188). Dashed lines indicate data from an individual animal while solid lines, columns and error bars show the mean ± SEM from n = 4 mice. One-way ANOVA followed by Tukey (b, c, e, f) and Sidak (h, i) post-hoc test; n.s., not significant; *p < 0.05 and **p < 0.01.
Extended Data Fig. 4 Increased colocalizations between microglial bulbous endings with VGAT puncta during anesthesia and emergence.
a, Schematic diagrams for colocalization analysis of microglial bulbous ending (IBA1, green) and a synapse marker (red, VGAT or VGLUT1) in confocal microscopy images. b, Representative images of microglia (IBA1, green) and GABAergic inhibitory synapses (VGAT, red) before, during, and after anesthesia. c, Quantification of IBA1+ and VGAT+ colocalized area (μm2) in imaging fields at each time point (Awake versus Isoflurane, p < 0.0001; Awake versus Emergence, p = 0.0121; Isoflurane versus Recovery, p < 0.0001). n = 80 (Awake), n = 215 (Isoflurane), n = 124 (Emergence) bulbous endings. d, Representative images of microglia (IBA1, green) and glutamatergic excitatory synapses (VGLUT1, red) before, during, and after anesthesia. e, Graph shows IBA1+ and VGLUT1+ colocalized area (μm2) in imaging fields at each time point(Awake versus Isoflurane, p = 0.9419; Awake versus Emergence, p = 0.0002; Isoflurane versus Recovery, p < 0.0001). n = 37 (Awake), n = 58 (Isoflurane), n = 80 (Emergence) bulbous endings. f, Representative images of microglia (Cx3cr1-GFP, green) and GABAergic inhibitory synapses (VGAT, red) before, during, and after anesthesia. g, Quantification of GFP+ and VGAT+ colocalized area (μm2) in imaging fields at each time point (Awake versus Isoflurane, p < 0.0001; Awake versus Emergence, p < 0.0001; Isoflurane versus Recovery, p < 0.3009). n = 44 (Awake), n = 95 (Isoflurane), n = 96 (Emergence) bulbous endings. h, Quantification of microglial bulbous endings contacting NeuN across experimental phases (Awake versus Isoflurane, p = 0.0013; Awake versus Emergence, p = 0.0202; Isoflurane versus Recovery, p = 0.2473). Awake, Isoflurane: n = 5, Emergence: n = 6 mice. i, Confocal imaging of representative soma-soma interaction between GCaMP+ neurons (blue) and microglia (green). PV+ boutons (red) were also shown. Diagram illustrates the observed phenomenon (right). j, Graph shows percentage of soma-soma interaction between microglia and neurons for all neuronal populations in the imaging field before, during, and after anesthesia (Awake versus Isoflurane, p = 0.8384; Awake versus Emergence, p = 0.9262; Isoflurane versus Recovery, p = 0.9791). n = 5 mice. Each point indicates data from an individual bulbous ending interaction (c, e, g) or mouse (h, j) while bars and error bars indicate the mean ± SEM. One-way ANOVA followed by Tukey post-hoc test; n.s., not significant; *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001. Scale bar: 20 μm or 2 μm (inset) (b, d, f), 5 μm (i).
Extended Data Fig. 5 Three-dimensional analysis of inhibitory synapses with microglial processes.
a, Representative 3D-image of co-immunostaining with VGAT (red) and gephyrin (cyan). Scale bar: 20 μm. b, Typical images of VGAT (red), gephyrin (cyan) and IBA1 microglial processes (green) before, during, and after anesthesia. Arrowheads: shielded VGAT+ synapse by microglia. Scale bar: 5 μm. c, Diagram showing the criteria for gephyrin+ VGAT+ synapse; double-positive was defined as when they were within 2 μm of each other in 3D images. d, Graph shows IBA1+ microglial process colocalized gephyrin+ VGAT+ synapses (%) at each time point (awake, isoflurane and emergence 30 min. Awake versus Isoflurane, p = 0.1117; Awake versus Emergence, p = 0.0038; Isoflurane versus Recovery, p = 0.6483). n = 49 (Awake), n = 35 (Isoflurane), n = 57 (Emergence) neurons from 5 mice. e, Representative immunofluorescent image of Kv2.1 (cyan), IBA1 microglial processes (yellow), NeuN nuclei (white), and VGAT puncta (magenta) in cortical tissue. Scale bar: 2 μm. f, Orthogonal view image shows bulbous ending co-localizing with VGAT close to Kv2.1 (same region with e). Scale bar: 2 μm. g, Graph shows quantification of Kv2.1+ cluster distribution. Kv2.1 signal is significantly higher within the contact site than non-contact site (Awake:non-contact versus Awake:contact, p = 0.0007; Isoflurane:non-contact versus Isoflurane:contact, p < 0.0001; Emergence:non-contact versus Emergence:contact, p = 0.1337). n = 5 mice. h, Graph shows quantification of Kv2.1+ VGAT+ cluster distribution. Colocalization is significantly higher within the contact site during anesthesia and emergence (Awake:non-contact versus Awake:contact, p = 0.9969; Isoflurane:non-contact versus Isoflurane:contact, p < 0.0001; Emergence:non-contact versus Emergence:contact, p = 0.0047). In all graphs, each point indicates data from an individual neuron while columns with error bars indicate the mean ± SEM. One-way ANOVA (d), two-way ANOVA (g,h) followed by Tukey post-hoc test; n.s., not significant; *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001.
Extended Data Fig. 6 GABAB receptor is enriched in the microglial bulbous endings during anesthesia.
a, Representative images of co-immunostaining with IBA1 (green) and GABAB1 receptor (GABAB1R, magenta) during anesthesia (30 min). Merged images of only co-localized GABAB1R are shown in the lower panel. Arrowheads: GABAB1R colocalized bulbous endings. Scale bar: 10 μm. b, Graph shows the percentage of GABAB1R+ microglia in somatosensory cortex and hippocampus. N = 5 mice. c, Representative orthogonal view image of GABAB1R+ microglial bulbous ending. Scale bar: 2 μm. d, Graph shows distribution of GABAB1R calculated by colocalized fluorescent signal; n = 23 (Soma), n = 28 (Process), n = 47 (Bulbous ending) from 4 mice at t = 30 min after the start of anesthesia (Soma versus Process, p = 0.0007; Soma versus Bulbous ending, p < 0.0001; Process versus Bulbous ending, p = 0.0015 e, Representative images of co-localized GABAB1R at each time point. Arrowheads: co-localized GABAB1R signal. Scale bar: 10 μm. f, Graph shows co-localized GABAB1R signal with bulbous endings at each time point [awake, isoflurane (at t = 30 min after the start of anesthesia), and emergence (at t = 30 min after termination of anesthesia)]. Awake versus Isoflurane, p = 0.0129; Awake versus Emergence, p = 0.8327; Isoflurane versus Recovery, p = 0.0008; n = 34 (Awake), n = 39 (Isoflurane), n = 48 (Emergence) from 4 mice. g, Graph shows co-localized GABAB1R signal with microglial soma at each time point (Awake versus Isoflurane, p = 0.0005; Awake versus Emergence, p = 0.4749; Isoflurane versus Recovery, p = 0.0102); n = 23 (Awake), n = 24 (Isoflurane), n = 29 (Emergence) from 4 mice. Each point indicates data from an individual mouse (b) or region of interest (ROI) (d, f, g), while bars and error bars indicate the mean ± SEM. One-way ANOVA followed by Tukey post-hoc test; *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001.
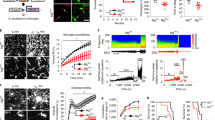
Extended Data Fig. 7 Near-infrared branding to correlate two-photon images with electron microscopy and study microglia-neuron interaction.
a, Timeline of experimental procedures necessary to capture an EM volume from the site of two-photon live imaging. Following in vivo two-photon imaging, brain tissue was fixed. We then re-identified the same region of imaging in fixed tissue under the two-photon microscope and performed near-infrared branding to create defined fiducial marks. This region was further cut down and processed for EM imaging, including tissue embedding and dehydration. Serial block-face scanning electron microscopy (SBF-SEM) was performed on sequential sections using the fiducial marks from laser branding to ensure proper tissue orientation. The image was reconstructed for microglial bulbous ending interactions with PV boutons and neuronal somata at the ultrastructural level. For details, please see the methods section. b, Two-photon in vivo imaging of microglia-PV bouton interactions adjacent to a neuronal soma, which was followed by electron microscopy reconstruction. The boxed area was then observed by electron microscopy. Arrowhead: interaction site. Dashed line: region of interest (ROI) for Ca2+ activity analysis. c, Neuronal Ca2+ activity (∆F/F) before anesthesia (baseline) and during emergence. d, 3-D serial reconstruction of a microglial process, PV bouton with vesicles, neuronal soma at the ultrastructural level using SEM. White dashed line: representative slice shown in (e). e, Electron microscopic image showing that a microglial process shields a PV bouton.
Extended Data Fig. 8 Chemogenetic inhibition of PV neurons masked emergence-induced neuronal hyperactivity.
a, Experimental timeline of CNO treatment and two-photon imaging of neuronal Ca2+ activity. WT, PV-GiDREADD+ mice or PLX treated PV-GiDREADD+ mice were infected with AAV9.CaMKII.GCaMP6s and AAV2-hSyn-DIO-hM4D(Gi)-mCherry. b, Representative image of PVCre/+ mice expressing Gi-DREADD (on PV neurons, mCherry) and GCaMP6 (on excitatory neurons). Scale bar: 50 μm. c, Representative traces of neuronal Ca2+ activity (∆F/F) with chemogenetic inhibition of PV neurons by CNO before, during and after anesthesia in 3 groups: WT-GiDREADD+ mice, PV-GiDREADD+ mice or PLX treated PV-GiDREADD+ mice. d–f, Neuronal Ca2+ signal amplitude (d), time active (e) and signal area (f) across experimental time points for WT-GiDREADD+ mice (n = 3 mice), PV-GiDREADD+ mice (n = 4 mice) or PLX treated PV-GiDREADD+ mice (n = 5 mice). p = 0.0024 (WT-GiDREADD+ mice versus PV-GiDREADD+ mice), p = 0.0003 (WT-GiDREADD+ mice versus PV-GiDREADD+ mice with PLX), F(2, 90) = 9.024 (d). p < 0.0001 (WT-GiDREADD+ mice versus PV-GiDREADD+ mice), p = 0.0032 (WT-GiDREADD+ mice versus PV-GiDREADD+ mice with PLX), F(2, 90) = 12.62 (e). p = 0.0044 (WT-GiDREADD+ mice versus PV-GiDREADD+ mice), p = 0.4894 (WT-GiDREADD+ mice versus PV-GiDREADD+ mice with PLX), F(2, 90) = 5.835 (f). Each dashed line indicates data from an individual mouse, while solid lines and error bars show the mean ± SEM. Two-way ANOVA followed by Tukey post-hoc test; n.s., not significant; **p < 0.01 and ****p < 0.0001.
Extended Data Fig. 9 Effect of β2-adrenergic receptor agonist on microglial process dynamics and neuronal activity after anesthesia.
a, Experimental timeline of agonist administration and two-photon imaging. Formoterol was injected intraperitoneally (50 μg/kg) 30 minutes before two-photon imaging. b, Representative images of microglial morphology during each experimental phase (Scale bar: 10 μm). c, Time-course of changes in microglial surveillance territory (Vehicle: n = 6 mice, Formoterol: n = 5 mice). p = 0.0012 (Emergence 15 min, Adrb2 WT versus Adrb2 cKO), p < 0.0001 (Emergence 30 min), p = 0.0240 (Emergence 45 min), p = 0.3669 (Emergence 60 min), F(1, 63) = 46.76. d–f, Neuronal Ca2+ signal amplitude (d), time active (e) and signal area (f) across experimental time points for vehicle or formoterol treated mice (n = 7 mice for both groups). p = 0.0012 (Emergence 15 min, Adrb2 WT versus Adrb2 cKO), p < 0.0001 (Emergence 30 min), p = 0.0240 (Emergence 45 min), p = 0.3669 (Emergence 60 min), F(1, 72) = 37.52 (d). p = 0.0172 (Emergence 15 min, Adrb2 WT versus Adrb2 cKO), p = 0.0003 (Emergence 30 min), p = 0.1799 (Emergence 45 min), p = 0.9582 (Emergence 60 min), F(1, 72) = 14.38 (e). p = 0.0055 (Emergence 15 min, Adrb2 WT versus Adrb2 cKO), p < 0.0001 (Emergence 30 min), p = 0.0377 (Emergence 45 min), p = 0.9978 (Emergence 60 min), F(1, 72) = 22.52 (f). Dashed lines indicate data from an individual mouse. Solid lines represent the mean ± SEM. Two-way ANOVA followed by Tukey post-hoc test; n.s., not significant; *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001.
Extended Data Fig. 10 β2-adrenergic receptors in microglial process dynamics and neuronal activity.
a, A representative image of microglia with dynamic processes (left). The illustration describes the calculation of microglial process moving outward in its trajectory. Microglial process directness was calculated as the Euclidean distance of the process tips divided by its accumulated distance. b, Graph shows increasing process trajectory directness during anesthesia in Adrb2 WT mice but not in Adrb2 cKO mice. Each dashed line indicates data from individual microglia: n = 12 WT microglia and n = 10 cKO microglia from 5 mice. p = 0.0213, F(1, 40) = 4.442 (Adrb2 WT, Awake versus Isoflurane), p = 0.8694 (Adrb2 cKO, Awake versus Isoflurane), p < 0.0001, F(1, 40) = 31.70 (Awake, Adrb2 WT versus Adrb2 cKO), p = 0.0329 (Isoflurane, Adrb2 WT versus Adrb2 cKO). c, Diagrams of EEG electrode implantation and the study timeline. d, Power in the alpha, beta, theta, and delta frequency bands is expressed as a ratio of power in the emergence phase relative to the awake phase before and after recombination for Adrb2 cKO (n = 5 mice). p = 0.0031, t(8) = 4.167 (Alpha), p = 0.0131, t(8) = 3.172 (Beta), p = 0.0189, t(8) = 2.935 (Theta), p = 0.1121, t(8) = 1.785 (Delta). e, Area of co-labelling between IBA1 microglia and VGAT puncta across experimental phases and between genotypes. Each point indicates data from an individual animal while columns with error bars indicate the mean ± SEM (n = 5 mice). p = 0.0010 (Awake, Adrb2 WT versus Adrb2 cKO), p = 0.0027 (Isoflurane, Adrb2 WT versus Adrb2 cKO), p = 0.4360 (Emergence, Adrb2 WT versus Adrb2 cKO), F(1, 24) = 0.3449. Related to Fig. 7g. Two-way ANOVA followed by Sidak post-hoc test (b, e), paired t-test (d); n.s., not significant; *p < 0.05, **p < 0.01 and ****p < 0.0001.
Supplementary information
Supplementary Video 1
Calcium activity of excitatory neuron in somatosensory cortex increases after the cessation of anesthesia compared with the awake period. Related to Fig. 1c.
Supplementary Video 2
A raw image, single-cell and territory area are displayed after thresholding, in that order. Microglial territory area and number of bulbous endings increase during anesthesia. Related to Fig. 1l.
Supplementary Video 3
Representative timecourse images of microglial process dynamics before and after anesthesia (one frame per minute). Microglial territory area and number of bulbous endings increase during anesthesia.
Supplementary Video 4
Emergence-induced hyperactivity was suppressed after microglia ablation. Related to Fig. 2c.
Supplementary Video 5
Microglia, GFP (green); CaMKII+ neuron, tdTomato (red) and GCaMP6s (green). Arrowheads indicate bulbous endings in contact with the neuronal somata. Related to Fig. 3a.
Supplementary Video 6
Microglia, GFP (green); CaMKII+ neuron, GCaMP6s (green) and PV bouton, tdTomato (red). White circle outlines neuronal somata. Arrowheads indicate contact sites. PV bouton remains after retraction of the microglial process. Related to Fig. 4h.
Supplementary Video 7
The process tips of microglia inserted themselves into a space between neuronal somata and PV boutons. The first half of the video displays microglia (green), PV boutons (red) and neuronal somata (blue); for simplicity, the second half shows only shows microglia and PV boutons. Related to Fig. 5d.
Supplementary Video 8
NE concentration decreased in the parenchyma of Layer II/III somatosensory cortex during anesthesia but increased above awake baseline levels in the first 30 min of emergence. Related to Fig. 6b.
Supplementary Video 9
Representative images show that increased microglial process dynamics were lost in Adrb2 cKO mice. Related to Fig. 6f.
Supplementary Video 10
Representative images show emergence-induced hyperactivity exists before knockout of Adrb2 but is lost in Adrb2 cKO mice. Related to Fig. 7b,c.
Source data
Source Data Figures
Raw data for main Figs. 1–7.
Source Data Extended Data Figures
Raw data for Extended Data Figs. 1–10.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Haruwaka, K., Ying, Y., Liang, Y. et al. Microglia enhance post-anesthesia neuronal activity by shielding inhibitory synapses. Nat Neurosci 27, 449–461 (2024). https://doi.org/10.1038/s41593-023-01537-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-023-01537-8