Abstract
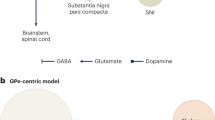
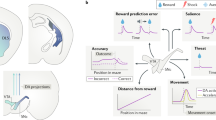
The striatal and pallidal complexes are basal ganglia structures that orchestrate learning and execution of flexible behavior. Models of how the basal ganglia subserve these functions have evolved considerably, and the advent of optogenetic and molecular tools has shed light on the heterogeneity of subcircuits within these pathways. However, a synthesis of how molecularly diverse neurons integrate into existing models of basal ganglia function is lacking. Here, we provide an overview of the neurochemical and molecular diversity of striatal and pallidal neurons and synthesize recent circuit connectivity studies in rodents that takes this diversity into account. We also highlight anatomical organizational principles that distinguish the dorsal and ventral basal ganglia pathways in rodents. Future work integrating the molecular and anatomical properties of striatal and pallidal subpopulations may resolve controversies regarding basal ganglia network function.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Albin, R. L., Young, A. B. & Penney, J. B. The functional anatomy of basal ganglia disorders. Trends Neurosci. 12, 366–375 (1989).
DeLong, M. R. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 13, 281–285 (1990).
Alexander, G. E. & Crutcher, M. D. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 13, 266–271 (1990).
Mink, J. W. Basal ganglia mechanisms in action selection, plasticity, and dystonia. Eur. J. Paediatr. Neurol. 22, 225–229 (2018).
Eisinger, R. S., Cernera, S., Gittis, A., Gunduz, A. & Okun, M. S. A review of basal ganglia circuits and physiology: application to deep brain stimulation. Parkinsonism Relat. Disord. 59, 9–20 (2019).
Frank, M. J. Computational models of motivated action selection in corticostriatal circuits. Curr. Opin. Neurobiol. 21, 381–386 (2011).
Gerfen, C. R. & Surmeier, D. J. Modulation of striatal projection systems by dopamine. Annu. Rev. Neurosci. 34, 441–466 (2011).
Tepper, J. M. et al. Heterogeneity and diversity of striatal GABAergic interneurons: update 2018. Front. Neuroanat. 12, 91 (2018).
Chen, R. et al. Decoding molecular and cellular heterogeneity of mouse nucleus accumbens. Nat. Neurosci. 24, 1757–1771 (2021).
Gokce, O. et al. Cellular taxonomy of the mouse striatum as revealed by single-cell RNA-seq. Cell Rep. 16, 1126–1137 (2016).
Kawaguchi, Y., Wilson, C. J. & Emson, P. C. Projection subtypes of rat neostriatal matrix cells revealed by intracellular injection of biocytin. J. Neurosci. 10, 3421–3438 (1990).
Calhoon, G. G. & O’Donnell, P. Closing the gate in the limbic striatum: prefrontal suppression of hippocampal and thalamic inputs. Neuron 78, 181–190 (2013).
Sippy, T., Lapray, D., Crochet, S. & Petersen, C. C. H. Cell-type-specific sensorimotor processing in striatal projection neurons during goal-directed behavior. Neuron 88, 298–305 (2015).
Du, K. et al. Cell-type-specific inhibition of the dendritic plateau potential in striatal spiny projection neurons. Proc. Natl Acad. Sci. USA 114, E7612–E7621 (2017).
Plotkin, J. L., Day, M. & Surmeier, D. J. Synaptically driven state transitions in distal dendrites of striatal spiny neurons. Nat. Neurosci. 14, 881–888 (2011).
Carter, A. G. & Sabatini, B. L. State-dependent calcium signaling in dendritic spines of striatal medium spiny neurons. Neuron 44, 483–493 (2004).
Carter, A. G., Soler-Llavina, G. J. & Sabatini, B. L. Timing and location of synaptic inputs determine modes of subthreshold integration in striatal medium spiny neurons. J. Neurosci. 27, 8967–8977 (2007).
Prager, E. M. et al. Dopamine oppositely modulates state transitions in striosome and matrix direct pathway striatal spiny neurons. Neuron 108, 1091–1102 (2020).
Pennartz, C. M., Groenewegen, H. J. & Lopes da Silva, F. H. The nucleus accumbens as a complex of functionally distinct neuronal ensembles: an integration of behavioural, electrophysiological and anatomical data. Prog. Neurobiol. 42, 719–761 (1994).
Hunnicutt B. J. et al. A comprehensive excitatory input map of the striatum reveals novel functional organization. eLife 5, e19103 (2016).
Haber, S. N. Corticostriatal circuitry. Dialogues Clin. Neurosci. 18, 7–21 (2016).
Peters, A. J., Fabre, J. M. J., Steinmetz, N. A., Harris, K. D. & Carandini, M. Striatal activity topographically reflects cortical activity. Nature 591, 420–425 (2021).
Stefanik, M. T. et al. Optogenetic inhibition of cocaine seeking in rats. Addict. Biol. 18, 50–53 (2013).
Hart, G., Bradfield, L. A., Fok, S. Y., Chieng, B. & Balleine, B. W. The bilateral prefronto-striatal pathway is necessary for learning new goal-directed actions. Curr. Biol. 28, 2218–2229 (2018).
Pascoli, V. et al. Contrasting forms of cocaine-evoked plasticity control components of relapse. Nature 509, 459–464 (2014).
Matsumoto, N., Minamimoto, T., Graybiel, A. M. & Kimura, M. Neurons in the thalamic CM-Pf complex supply striatal neurons with information about behaviorally significant sensory events. J. Neurophysiol. 85, 960–976 (2001).
Parker, P. R. L., Lalive, A. L. & Kreitzer, A. C. Pathway-specific remodeling of thalamostriatal synapses in parkinsonian mice. Neuron 89, 734–740 (2016).
Meffre, J. et al. Orexin in the posterior paraventricular thalamus mediates hunger-related signals in the nucleus accumbens Core. Curr. Biol. 29, 3298–3306 (2019).
Otis, J. M. et al. Paraventricular thalamus projection neurons integrate cortical and hypothalamic signals for cue-reward processing. Neuron 103, 423–431 (2019).
Britt, J. P. et al. Synaptic and behavioral profile of multiple glutamatergic inputs to the nucleus accumbens. Neuron 76, 790–803 (2012).
LeGates, T. A. et al. Reward behaviour is regulated by the strength of hippocampus-nucleus accumbens synapses. Nature 564, 258–262 (2018).
Yang, A. K., Mendoza, J. A., Lafferty, C. K., Lacroix, F. & Britt, J. P. Hippocampal input to the nucleus accumbens shell enhances food palatability. Biol. Psychiatry 87, 597–608 (2020).
Stuber, G. D. et al. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature 475, 377–380 (2011).
Beyeler, A. et al. Organization of valence-encoding and projection-defined neurons in the basolateral amygdala. Cell Rep. 22, 905–918 (2018).
van Holstein, M., MacLeod, P. E. & Floresco, S. B. Basolateral amygdala - nucleus accumbens circuitry regulates optimal cue-guided risk/reward decision making. Prog. Neuropsychopharmacol. Biol. Psychiatry 98, 109830 (2020).
Wall, N. R., De La Parra, M., Callaway, E. M. & Kreitzer, A. C. Differential innervation of direct- and indirect-pathway striatal projection neurons. Neuron 79, 347–360 (2013).
Lu, J. et al. Whole-brain mapping of direct inputs to dopamine D1 and D2 receptor-expressing medium spiny neurons in the posterior dorsomedial striatum. eNeuro https://www.eneuro.org/content/8/1/ENEURO.0348-20.2020 (2021).
Doig, N. M., Moss, J. & Bolam, J. P. Cortical and thalamic innervation of direct and indirect pathway medium-sized spiny neurons in mouse striatum. J. Neurosci. 30, 14610–14618 (2010).
Barrientos, C. et al. Cocaine-induced structural plasticity in input regions to distinct cell types in nucleus accumbens. Biol. Psychiatry 84, 893–904 (2018).
Johansson, Y. & Silberberg, G. The functional organization of cortical and thalamic inputs onto five types of striatal neurons is determined by source and target cell identities. Cell Rep. 30, 1178–1194 (2020).
Baimel, C., McGarry, L. M. & Carter, A. G. The projection targets of medium spiny neurons govern cocaine-evoked synaptic plasticity in the nucleus accumbens. Cell Rep. 28, 2256–2263 (2019).
MacAskill, A. F., Little, J. P., Cassel, J. M. & Carter, A. G. Subcellular connectivity underlies pathway-specific signaling in the nucleus accumbens. Nat. Neurosci. 15, 1624–1626 (2012).
French, S. J. & Totterdell, S. Hippocampal and prefrontal cortical inputs monosynaptically converge with individual projection neurons of the nucleus accumbens. J. Comp. Neurol. 446, 151–165 (2002).
Gittis, A. H., Nelson, A. B., Thwin, M. T., Palop, J. J. & Kreitzer, A. C. Distinct roles of GABAergic interneurons in the regulation of striatal output pathways. J. Neurosci. 30, 2223–2234 (2010).
Mallet, N., Moine, C. L., Charpier, S. & Gonon, F. Feedforward inhibition of projection neurons by fast-spiking GABA interneurons in the rat striatum in vivo. J. Neurosci. 25, 3857–3869 (2005).
Berke, J. D., Okatan, M., Skurski, J. & Eichenbaum, H. B. Oscillatory entrainment of striatal neurons in freely moving rats. Neuron 43, 883–896 (2004).
Koós, T. & Tepper, J. M. Inhibitory control of neostriatal projection neurons by GABAergic interneurons. Nat. Neurosci. 2, 467–472 (1999).
Gittis, A. H. et al. Rapid target-specific remodeling of fast-spiking inhibitory circuits after loss of dopamine. Neuron 71, 858–868 (2011).
Lee, K. et al. Parvalbumin interneurons modulate striatal output and enhance performance during associative learning. Neuron 93, 1451–1463 (2017).
Owen, S. F., Berke, J. D. & Kreitzer, A. C. Fast-spiking interneurons supply feed-forward control of bursting, calcium, and plasticity for efficient learning. Cell 172, 683–695 (2018).
Fukuda, T. Network architecture of gap junction-coupled neuronal linkage in the striatum. J. Neurosci. 29, 1235–1243 (2009).
Berke, J. D. Functional properties of striatal fast-spiking interneurons. Front. Syst. Neurosci. 5, 45 (2011).
Burke, D. A., Rotstein, H. G. & Alvarez, V. A. Striatal local circuitry: a new framework for lateral inhibition. Neuron 96, 267–284 (2017).
Baimel, C., Jang, E., Scudder, S. L., Manoocheri, K. & Carter, A. G. Hippocampal-evoked inhibition of cholinergic interneurons in the nucleus accumbens. Cell Rep. 40, 111042 (2022).
Straub, C. et al. Principles of synaptic organization of GABAergic interneurons in the striatum. Neuron 92, 84–92 (2016).
Oldenburg, I. A. & Ding, J. B. Cholinergic modulation of synaptic integration and dendritic excitability in the striatum. Curr. Opin. Neurobiol. 21, 425–432 (2011).
Kramer, P. F. et al. Synaptic-like axo-axonal transmission from striatal cholinergic interneurons onto dopaminergic fibers. Neuron 110, 2949–2960 (2022).
Threlfell, S. et al. Striatal dopamine release is triggered by synchronized activity in cholinergic interneurons. Neuron 75, 58–64 (2012).
Bennett, B. D. & Bolam, J. P. Synaptic input and output of parvalbumin-immunoreactive neurons in the neostriatum of the rat. Neuroscience 62, 707–719 (1994).
Klug, J. R. et al. Differential inputs to striatal cholinergic and parvalbumin interneurons imply functional distinctions. eLife 7, e35657 (2018).
Gage, G. J., Stoetzner, C. R., Wiltschko, A. B. & Berke, J. D. Selective activation of striatal fast-spiking interneurons during choice execution. Neuron 67, 466–479 (2010).
Mallet, N. et al. Dichotomous organization of the external globus pallidus. Neuron 74, 1075–1086 (2012).
Mallet, N. et al. Arkypallidal cells send a stop signal to striatum. Neuron 89, 308–316 (2016).
Vachez, Y. M. et al. Ventral arkypallidal neurons inhibit accumbal firing to promote reward consumption. Nat. Neurosci. 24, 379–390 (2021).
Straub, C., Tritsch, N. X., Hagan, N. A., Gu, C. & Sabatini, B. L. Multiphasic modulation of cholinergic interneurons by nigrostriatal afferents. J. Neurosci. 34, 8557–8569 (2014).
Brown, M. T. C. et al. Ventral tegmental area GABA projections pause accumbal cholinergic interneurons to enhance associative learning. Nature 492, 452–456 (2012).
van Zessen, R., Phillips, J. L., Budygin, E. A. & Stuber, G. D. Activation of VTA GABA neurons disrupts reward consumption. Neuron 73, 1184–1194 (2012).
Al-Hasani, R. et al. Ventral tegmental area GABAergic inhibition of cholinergic interneurons in the ventral nucleus accumbens shell promotes reward reinforcement. Nat. Neurosci. 24, 1414–1428 (2021).
Miranda-Barrientos, J. et al. Ventral tegmental area GABA, glutamate, and glutamate-GABA neurons are heterogeneous in their electrophysiological and pharmacological properties. Eur. J. Neurosci. https://doi.org/10.1111/ejn.15156 (2021).
Root, D. H. et al. Distinct signaling by ventral tegmental area glutamate, gaba, and combinatorial glutamate-GABA neurons in motivated behavior. Cell Rep. 32, 108094 (2020).
Zych, S. M. & Ford, C. P. Divergent properties and independent regulation of striatal dopamine and GABA co-transmission. Cell Rep. 39, 110823 (2022).
Kravitz, A. V. et al. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature 466, 622–626 (2010).
Kravitz, A. V., Tye, L. D. & Kreitzer, A. C. Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nat. Neurosci. 15, 816–818 (2012).
London, T. D. et al. Coordinated ramping of dorsal striatal pathways preceding food approach and consumption. J. Neurosci. 38, 3547–3558 (2018).
Tecuapetla, F., Jin, X., Lima, S. Q. & Costa, R. M. Complementary contributions of striatal projection pathways to action initiation and execution. Cell 166, 703–715 (2016).
Mink, J. W. The basal ganglia: focused selection and inhibition of competing motor programs. Prog. Neurobiol. 50, 381–425 (1996).
Parker, J. G. et al. Diametric neural ensemble dynamics in parkinsonian and dyskinetic states. Nature 557, 177–182 (2018).
Barbera, G. et al. Spatially compact neural clusters in the dorsal striatum encode locomotion relevant information. Neuron 92, 202–213 (2016).
Klaus, A. et al. The spatiotemporal organization of the striatum encodes action space. Neuron 95, 1171–1180 (2017).
Bariselli, S., Fobbs, W. C., Creed, M. C. & Kravitz, A. V. A competitive model for striatal action selection. Brain Res. 1713, 70–79 (2019).
Taverna, S., Ilijic, E. & Surmeier, D. J. Recurrent collateral connections of striatal medium spiny neurons are disrupted in models of parkinson’s disease. J. Neurosci. 28, 5504–5512 (2008).
Cepeda, C. et al. Differential electrophysiological properties of dopamine D1 and D2 receptor-containing striatal medium-sized spiny neurons. Eur. J. Neurosci. 27, 671–682 (2008).
Willett, J. A. et al. Electrophysiological properties of medium spiny neuron subtypes in the caudate-putamen of prepubertal male and female Drd1a-tdTomato line 6 BAC transgenic mice. eNeuro https://www.eneuro.org/content/6/2/eneuro.0016-19.2019 (2019).
Cui, G. et al. Concurrent activation of striatal direct and indirect pathways during action initiation. Nature 494, 238–242 (2013).
Geddes, C. E., Li, H. & Jin, X. Optogenetic editing reveals the hierarchical organization of learned action sequences. Cell 174, 32–43 (2018).
Zalocusky, K. A. et al. Nucleus accumbens D2R cells signal prior outcomes and control risky decision-making. Nature 531, 642–646 (2016).
Cohen, M. X. & Frank, M. J. Neurocomputational models of basal ganglia function in learning, memory and choice. Behav. Brain Res. 199, 141–156 (2009).
Girault, J. A. Integrating neurotransmission in striatal medium spiny neurons. Adv. Exp. Med. Biol. https://doi.org/10.1007/978-3-7091-0932-8_18(2012).
Pacheco-Cano, M. T., Bargas, J., Hernández-López, S., Tapia, D. & Galarraga, E. Inhibitory action of dopamine involves a subthreshold Cs+-sensitive conductance in neostriatal neurons. Exp. Brain Res. 110, 205–211 (1996).
Zhao, B. et al. Differential dopaminergic regulation of inwardly rectifying potassium channel mediated subthreshold dynamics in striatal medium spiny neurons. Neuropharmacology 107, 396–410 (2016).
Podda, M. V., Riccardi, E., D’Ascenzo, M., Azzena, G. B. & Grassi, C. Dopamine D1-like receptor activation depolarizes medium spiny neurons of the mouse nucleus accumbens by inhibiting inwardly rectifying K+ currents through a cAMP-dependent protein kinase A-independent mechanism. Neuroscience 167, 678–690 (2010).
Nisenbaum, E. S. & Wilson, C. J. Potassium currents responsible for inward and outward rectification in rat neostriatal spiny projection neurons. J. Neurosci. 15, 4449–4463 (1995).
Lahiri, A. K. & Bevan, M. D. Dopaminergic transmission rapidly and persistently enhances excitability of D1 receptor-expressing striatal projection neurons. Neuron 106, 277–290 (2020).
Surmeier, D. J., Bargas, J., Hemmings, H. C., Nairn, A. C. & Greengard, P. Modulation of calcium currents by a D1 dopaminergic protein kinase/phosphatase cascade in rat neostriatal neurons. Neuron 14, 385–397 (1995).
Surmeier, D. J. & Kitai, S. T. D1 and D2 dopamine receptor modulation of sodium and potassium currents in rat neostriatal neurons. Prog. Brain Res. 99, 309–324 (1993).
Yagishita, S. et al. A critical time window for dopamine actions on the structural plasticity of dendritic spines. Science 345, 1616–1620 (2014).
Mangiavacchi, S. & Wolf, M. E. D1 dopamine receptor stimulation increases the rate of AMPA receptor insertion onto the surface of cultured nucleus accumbens neurons through a pathway dependent on protein kinase A. J. Neurochem. 88, 1261–1271 (2004).
Shen, W., Flajolet, M., Greengard, P. & Surmeier, D. J. Dichotomous dopaminergic control of striatal synaptic plasticity. Science 321, 848–851 (2008).
Kreitzer, A. C. & Malenka, R. C. Dopamine modulation of state-dependent endocannabinoid release and long-term depression in the striatum. J. Neurosci. 25, 10537–10545 (2005).
Lemos, J. C. et al. Enhanced GABA transmission drives bradykinesia following loss of dopamine D2 receptor signaling. Neuron 90, 824–838 (2016).
Dobbs, L. K. et al. Dopamine regulation of lateral inhibition between striatal neurons gates the stimulant actions of cocaine. Neuron 90, 1100–1113 (2016).
Creed, M., Ntamati, N. R., Chandra, R., Lobo, M. K. & Lüscher, C. Convergence of reinforcing and anhedonic cocaine effects in the ventral pallidum. Neuron 92, 214–226 (2016).
Cazorla, M. et al. Dopamine D2 receptors regulate the anatomical and functional balance of basal ganglia circuitry. Neuron 81, 153–164 (2014).
Aristieta et al. A disynaptic circuit in the globus pallidus controls locomotion inhibition. Curr. Biol. 31, 707–721 (2021).
Cole, S. L., Robinson, M. J. F. & Berridge, K. C. Optogenetic self-stimulation in the nucleus accumbens: D1 reward versus D2 ambivalence. PLoS ONE 13, e0207694 (2018).
Wu, Y., Richard, S. & Parent, A. The organization of the striatal output system: a single-cell juxtacellular labeling study in the rat. Neurosci. Res. 38, 49–62 (2000).
Spix, T. A. et al. Population-specific neuromodulation prolongs therapeutic benefits of deep brain stimulation. Science 374, 201–206 (2021).
Cui, Q. et al. Striatal direct pathway targets Npas1+ pallidal neurons. J. Neurosci. 41, 3966–3987 (2021).
O’Connor, E. C. et al. Accumbal D1R neurons projecting to lateral hypothalamus authorize feeding. Neuron 88, 553–564 (2015).
Bocklisch, C. et al. Cocaine disinhibits dopamine neurons by potentiation of GABA transmission in the ventral tegmental area. Science 341, 1521–1525 (2013).
Gallo, E. F. et al. Upregulation of dopamine D2 receptors in the nucleus accumbens indirect pathway increases locomotion but does not reduce alcohol consumption. Neuropsychopharmacology 40, 1609–1618 (2015).
Wang, L. et al. Optogenetic activation of GABAergic neurons in the nucleus accumbens decreases the activity of the ventral pallidum and the expression of cocaine-context-associated memory. Int. J. Neuropsychopharmacol. 17, 753–763 (2014).
Kupchik, Y. M. et al. Coding the direct/indirect pathways by D1 and D2 receptors is not valid for accumbens projections. Nat. Neurosci. 18, 1230–1232 (2015).
Pardo-Garcia, T. R. et al. Ventral pallidum Is the primary target for accumbens D1 projections driving cocaine seeking. J. Neurosci. 39, 2041–2051 (2019).
Tripathi, A., Prensa, L., Cebrián, C. & Mengual, E. Axonal branching patterns of nucleus accumbens neurons in the rat. J. Comp. Neurol. 518, 4649–4673 (2010).
Root, D. H. The ventromedial ventral pallidum subregion is necessary for outcome-specific Pavlovian-instrumental transfer. J. Neurosci. 33, 18707–18709 (2013).
Soares-Cunha, C. et al. Nucleus accumbens medium spiny neurons subtypes signal both reward and aversion. Mol. Psychiatry 25, 3241–3255 (2020).
Abdi, A. et al. Prototypic and arkypallidal neurons in the dopamine-intact external globus pallidus. J. Neurosci. 35, 6667–6688 (2015).
Flandin, P., Kimura, S. & Rubenstein, J. L. R. The progenitor zone of the ventral medial ganglionic eminence requires Nkx2-1 to generate most of the globus pallidus but few neocortical interneurons. J. Neurosci. 30, 2812–2823 (2010).
Butt, S. J. B. et al. The requirement of Nkx2-1 in the temporal specification of cortical interneuron subtypes. Neuron 59, 722–732 (2008).
Dodson, P. D. et al. Distinct developmental origins manifest in the specialized encoding of movement by adult neurons of the external globus pallidus. Neuron 86, 501–513 (2015).
Abecassis, Z. A. et al. Npas1+-Nkx2.1+ neurons are an integral part of the cortico-pallido-cortical loop. J. Neurosci. 40, 743–768 (2020).
Batista-Brito, R. et al. The cell-intrinsic requirement of Sox6 for cortical interneuron development. Neuron 63, 466–481 (2009).
Abrahao, K. P. & Lovinger, D. M. Classification of GABAergic neuron subtypes from the globus pallidus using wild-type and transgenic mice. J. Physiol. 596, 4219–4235 (2018).
Mastro, K. J., Bouchard, R. S., Holt, H. A. K. & Gittis, A. H. Transgenic mouse lines subdivide external segment of the globus pallidus (GPe) neurons and reveal distinct GPe output pathways. J. Neurosci. 34, 2087–2099 (2014).
Mastro, K. J. et al. Cell-specific pallidal intervention induces long-lasting motor recovery in dopamine depleted mice. Nat. Neurosci. 20, 815–823 (2017).
Hernández, V. M. et al. Parvalbumin+ neurons and Npas1+ neurons are distinct neuron classes in the mouse external globus pallidus. J. Neurosci. 35, 11830–11847 (2015).
Liodis, P. et al. Lhx6 activity is required for the normal migration and specification of cortical interneuron subtypes. J. Neurosci. 27, 3078–3089 (2007).
Filice, F., Vörckel, K. J., Sungur, A. Ö., Wöhr, M. & Schwaller, B. Reduction in parvalbumin expression not loss of the parvalbumin-expressing GABA interneuron subpopulation in genetic parvalbumin and shank mouse models of autism. Mol. Brain. 9, 10 (2016).
Caballero, A., Flores-Barrera, E., Thomases, D. R. & Tseng, K. Y. Downregulation of parvalbumin expression in the prefrontal cortex during adolescence causes enduring prefrontal disinhibition in adulthood. Neuropsychopharmacology 45, 1527–1535 (2020).
Lilascharoen, V. et al. Divergent pallidal pathways underlying distinct parkinsonian behavioral deficits. Nat. Neurosci. 24, 504–515 (2021).
Knowland, D. et al. Distinct ventral pallidal neural populations mediate separate symptoms of depression. Cell 170, 284–297 (2017).
Faget, L. et al. Opponent control of behavioral reinforcement by inhibitory and excitatory projections from the ventral pallidum. Nat. Commun. 9, 849 (2018).
Tooley, J. et al. Glutamatergic ventral pallidal neurons modulate activity of the habenula-tegmental circuitry and constrain reward seeking. Biol. Psychiatry 83, 1012–1023 (2018).
Stephenson-Jones, M. et al. Opposing contributions of GABAergic and glutamatergic ventral pallidal neurons to motivational behaviors. Neuron 105, 921–933 (2020).
Farrell, M. R. et al. Ventral pallidum GABA neurons mediate motivation underlying risky choice. J. Neurosci. 41, 4500–4513 (2021).
Mahler, S. V. et al. Designer receptors show role for ventral pallidum input to ventral tegmental area in cocaine seeking. Nat. Neurosci. 17, 577–585 (2014).
Heinsbroek, J. A. et al. Opposing regulation of cocaine seeking by glutamate and GABA neurons in the ventral pallidum. Cell Rep. 30, 2018–2027 (2020).
Wallace, M. L. et al. Genetically distinct parallel pathways in the entopeduncular nucleus for limbic and sensorimotor output of the basal ganglia. Neuron 94, 138–152 (2017).
Glajch, K. E. et al. Npas1+ pallidal neurons target striatal projection neurons. J. Neurosci. 36, 5472–5488 (2016).
Churchill, L. & Kalivas, P. W. A topographically organized gamma-aminobutyric acid projection from the ventral pallidum to the nucleus accumbens in the rat. J. Comp. Neurol. 345, 579–595 (1994).
Banghart, M. R., Neufeld, S. Q., Wong, N. C. & Sabatini, B. L. Enkephalin disinhibits mu opioid receptor-rich striatal patches via delta opioid receptors. Neuron 88, 1227–1239 (2015).
Al-Hasani, R. et al. In vivo detection of optically-evoked opioid peptide release. eLife 7, e36520 (2018).
Saunders, A., Huang, K. W. & Sabatini, B. L. Globus Pallidus externus neurons expressing parvalbumin interconnect the subthalamic nucleus and striatal interneurons. PLoS ONE 11, e0149798 (2016).
Gielow, M. R. & Zaborszky, L. The input–output relationship of the cholinergic basal forebrain. Cell Rep. 18, 1817–1830 (2017).
Gritton, H. J. et al. Cortical cholinergic signaling controls the detection of cues. Proc. Natl Acad. Sci. USA 113, E1089–E1097 (2016).
Saunders, A. et al. A direct GABAergic output from the basal ganglia to frontal cortex. Nature 521, 85–89 (2015).
Baxter, M. G. & Chiba, A. A. Cognitive functions of the basal forebrain. Curr. Opin. Neurobiol. 9, 178–183 (1999).
Bariselli, S., Miyazaki, N. L., Creed, M. C. & Kravitz, A. V. Orbitofrontal-striatal potentiation underlies cocaine-induced hyperactivity. Nat. Commun. 11, 3996 (2020).
Creed, M., Pascoli, V. J. & Lüscher, C. Addiction therapy. Refining deep brain stimulation to emulate optogenetic treatment of synaptic pathology. Science 347, 659–664 (2015).
Acknowledgements
This work was funded by the Brain and Behavior Research Foundation (grant no. 27197 to M.C.C.), Rita Allen Scholar Award in Pain (to M.C.C), the National Institutes on Drug Abuse (R01DA049924 and R01DA058755 to M.C.C.) and a Canadian Institutes of Health Research Michael Smith Foreign Study Supplement (to L.Z.F.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Neuroscience thanks Talia Lerner and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fang, L.Z., Creed, M.C. Updating the striatal–pallidal wiring diagram. Nat Neurosci 27, 15–27 (2024). https://doi.org/10.1038/s41593-023-01518-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-023-01518-x