Abstract
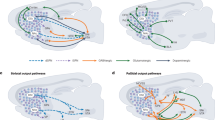
The external globus pallidus (GPe) of the basal ganglia has been underappreciated owing to poor understanding of its cells and circuits. It was assumed that the GPe consisted of a homogeneous neuron population primarily serving as a ‘relay station’ for information flowing through the indirect basal ganglia pathway. However, the advent of advanced tools in rodent models has sparked a resurgence in interest in the GPe. Here, we review recent data that have unveiled the cell and circuit complexity of the GPe. These discoveries have revealed that the GPe does not conform to traditional views of the basal ganglia. In particular, recent evidence confirms that the afferent and efferent connections of the GPe span both the direct and the indirect pathways. Furthermore, the GPe displays broad interconnectivity beyond the basal ganglia, consistent with its emerging multifaceted roles in both motor and non-motor functions. In summary, recent data prompt new proposals for computational rules of the basal ganglia.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Grillner, S. & El Manira, A. Current principles of motor control, with special reference to vertebrate locomotion. Physiol. Rev. 100, 271–320 (2020).
Wallace, M. L. et al. Genetically distinct parallel pathways in the entopeduncular nucleus for limbic and sensorimotor output of the basal ganglia. Neuron 94, 138–152 (2017).
Poulin, J.-F. et al. Mapping projections of molecularly defined dopamine neuron subtypes using intersectional genetic approaches. Nat. Neurosci. 21, 1260–1271 (2018).
Saunders, A. et al. Molecular diversity and specializations among the cells of the adult mouse brain. Cell 174, 1015–1030 (2018).
Welch, J. D. et al. Single-cell multi-omic integration compares and contrasts features of brain cell identity. Cell 177, 1873–1887 (2019).
Wallén-Mackenzie, Å. et al. Spatio-molecular domains identified in the mouse subthalamic nucleus and neighboring glutamatergic and GABAergic brain structures. Commun. Biol. 3, 338 (2020).
McElvain, L. E. et al. Specific populations of basal ganglia output neurons target distinct brain stem areas while collateralizing throughout the diencephalon. Neuron 109, 1721–1738 (2021).
Miyamoto, Y. & Fukuda, T. The habenula-targeting neurons in the mouse entopeduncular nucleus contain not only somatostatin-positive neurons but also nitric oxide synthase-positive neurons. Brain Struct. Funct. 226, 1497–1510 (2021).
Jeon, H. et al. Topographic connectivity and cellular profiling reveal detailed input pathways and functionally distinct cell types in the subthalamic nucleus. Cell Rep. 38, 110439 (2022).
Liu, D. et al. A common hub for sleep and motor control in the substantia nigra. Science 367, 440–445 (2020).
Mastro, K. J. et al. Cell-specific pallidal intervention induces long-lasting motor recovery in dopamine-depleted mice. Nat. Neurosci. 20, 815–823 (2017).
Glajch, K. E. et al. Npas1+ pallidal neurons target striatal projection neurons. J. Neurosci. 36, 5472–5488 (2016).
Pamukcu, A. et al. Parvalbumin+ and Npas1+ pallidal neurons have distinct circuit topology and function. J. Neurosci. 40, 7855–7876 (2020).
Cui, Q. et al. Dissociable roles of pallidal neuron subtypes in regulating motor patterns. J. Neurosci. 41, 4036–4059 (2021).
Aristieta, A. et al. A disynaptic circuit in the globus pallidus controls locomotion inhibition. Curr. Biol. 31, 707–721 (2021).
Lilascharoen, V. et al. Divergent pallidal pathways underlying distinct parkinsonian behavioral deficits. Nat. Neurosci. 24, 504–515 (2021).
Arkadir, D., Morris, G., Vaadia, E. & Bergman, H. Independent coding of movement direction and reward prediction by single pallidal neurons. J. Neurosci. 24, 10047–10056 (2004).
Yoshida, A. & Tanaka, M. Two types of neurons in the primate globus pallidus external segment play distinct roles in antisaccade generation. Cereb. Cortex 26, 1187–1199 (2016).
Gu, B. M., Schmidt, R. & Berke, J. D. Globus pallidus dynamics reveal covert strategies for behavioral inhibition. eLife 9, e57215 (2020).
Kita, H. Globus pallidus external segment. Prog. Brain Res. 160, 111–133 (2007).
Jaeger, D. & Kita, H. Functional connectivity and integrative properties of globus pallidus neurons. Neuroscience 198, 44–53 (2011).
Hegeman, D. J., Hong, E. S., Hernández, V. M. & Chan, C. S. The external globus pallidus: progress and perspectives. Eur. J. Neurosci. 43, 1239–1265 (2016).
Nóbrega-Pereira, S. et al. Origin and molecular specification of globus pallidus neurons. J. Neurosci. 30, 2824–2834 (2010).
Flandin, P., Kimura, S. & Rubenstein, J. L. R. The progenitor zone of the ventral medial ganglionic eminence requires Nkx2-1 to generate most of the globus pallidus but few neocortical interneurons. J. Neurosci. 30, 2812–2823 (2010).
Abdi, A. et al. Prototypic and arkypallidal neurons in the dopamine-intact external globus pallidus. J. Neurosci. 35, 6667–6688 (2015).
Dodson, P. D. et al. Distinct developmental origins manifest in the specialized encoding of movement by adult neurons of the external globus pallidus. Neuron 86, 501–513 (2015).
Hernández, V. M. et al. Parvalbumin+ neurons and Npas1+ neurons are distinct neuron classes in the mouse external globus pallidus. J. Neurosci. 35, 11830–11847 (2015).
Abecassis, Z. A. et al. Npas1+-Nkx2.1+ neurons are an integral part of the cortico–pallido–cortical loop. J. Neurosci. 40, 743–768 (2020).
Mastro, K. J., Bouchard, R. S., Holt, H. A. K. & Gittis, A. H. Transgenic mouse lines subdivide external segment of the globus pallidus (GPe) neurons and reveal distinct GPe output pathways. J. Neurosci. 34, 2087–2099 (2014).
Saunders, A. et al. A direct GABAergic output from the basal ganglia to frontal cortex. Nature 521, 85–89 (2015).
Higgs, M. H., Jones, J. A., Chan, C. S. & Wilson, C. J. Periodic unitary synaptic currents in the mouse globus pallidus during spontaneous firing in slices. J. Neurophysiol. 125, 1482–1500 (2021).
Mizutani, K., Takahashi, S., Okamoto, S., Karube, F. & Fujiyama, F. Substance P effects exclusively on prototypic neurons in mouse globus pallidus. Brain Struct. Funct. 222, 4089–4110 (2017).
Oh, Y.-M. et al. Using a novel PV-Cre rat model to characterize pallidonigral cells and their terminations. Brain Struct. Funct. 222, 2359–2378 (2017).
Abrahao, K. P. & Lovinger, D. M. Classification of GABAergic neuron subtypes from the globus pallidus using wild-type and transgenic mice. J. Physiol. 596, 4219–4235 (2018).
Saunders, A., Huang, K. W. & Sabatini, B. L. Globus pallidus externus neurons expressing parvalbumin interconnect the subthalamic nucleus and striatal interneurons. PloS ONE 11, e0149798 (2016).
Zeisel, A. et al. Molecular architecture of the mouse nervous system. Cell 174, 999–1014 (2018).
Katabi, S., Adler, A., Deffains, M. & Bergman, H. Dichotomous activity and function of neurons with low- and high-frequency discharge in the external globus pallidus of non-human primates. Cell Rep. 42, 111898 (2023).
Mallet, N. et al. Dichotomous organization of the external globus pallidus. Neuron 74, 1075–1086 (2012).
Ketzef, M. & Silberberg, G. Differential synaptic input to external globus pallidus neuronal subpopulations in vivo. Neuron 109, 516–529 (2021).
Fishell, G. & Kepecs, A. Interneuron types as attractors and controllers. Annu. Rev. Neurosci. 43, 1–30 (2020).
Long, J. E., Cobos, I., Potter, G. B. & Rubenstein, J. L. R. Dlx1&2 and Mash1 transcription factors control MGE and CGE patterning and differentiation through parallel and overlapping pathways. Cereb. Cortex 19, i96–i106 (2009).
Gelman, D. M. et al. The embryonic preoptic area is a novel source of cortical GABAergic interneurons. J. Neurosci. 29, 9380–9389 (2009).
Gelman, D. et al. A wide diversity of cortical GABAergic interneurons derives from the embryonic preoptic area. J. Neurosci. 31, 16570–16580 (2011).
Chen, Y.-J. J. et al. Single-cell RNA sequencing identifies distinct mouse medial ganglionic eminence cell types. Sci. Rep. 7, 45656 (2017).
Poulin, J.-F. et al. PRISM: a progenitor-restricted intersectional fate mapping approach redefines forebrain lineages. Dev. Cell 53, 740–753 (2020).
Hunt, A. J. et al. Paraventricular hypothalamic and amygdalar CRF neurons synapse in the external globus pallidus. Brain Struct. Funct. 223, 2685–2698 (2018).
Cui, Q. et al. Striatal direct pathway targets Npas1+ pallidal neurons. J. Neurosci. 41, 3966–3987 (2021).
Chang, S. et al. Tripartite extended amygdala-basal ganglia CRH circuit drives locomotor activation and avoidance behavior. Sci. Adv. 8, eabo1023 (2022).
Fujiyama, F. et al. Exclusive and common targets of neostriatofugal projections of rat striosome neurons: a single neuron-tracing study using a viral vector. Eur. J. Neurosci. 33, 668–677 (2011).
Wu, Y., Richard, S. & Parent, A. The organization of the striatal output system: a single-cell juxtacellular labeling study in the rat. Neurosci. Res. 38, 49–62 (2000).
Lévesque, M. & Parent, A. The striatofugal fiber system in primates: a reevaluation of its organization based on single-axon tracing studies. Proc. Natl Acad. Sci. USA 102, 11888–11893 (2005).
Cazorla, M. et al. Dopamine D2 receptors regulate the anatomical and functional balance of basal ganglia circuitry. Neuron 81, 153–164 (2014).
Okamoto, S. et al. Overlapping projections of neighboring direct and indirect pathway neostriatal neurons to globus pallidus external segment. iScience 23, 101409 (2020).
Foster, N. N. et al. The mouse cortico–basal ganglia–thalamic network. Nature 598, 188–194 (2021).
Yuan, X.-S. et al. Striatal adenosine A2A receptor neurons control active-period sleep via parvalbumin neurons in external globus pallidus. eLife 6, e29055 (2017).
Johansson, Y. & Ketzef, M. Sensory processing in external globus pallidus neurons. Cell Rep. 42, 111952 (2023).
Jones, J. A., Higgs, M. H., Olivares, E., Peña, J. & Wilson, C. J. Spontaneous activity of the local GABAergic synaptic network causes irregular neuronal firing in the external globus pallidus. J. Neurosci. 43, 1281–1297 (2023).
Sato, F., Parent, M., Levesque, M. & Parent, A. Axonal branching pattern of neurons of the subthalamic nucleus in primates. J. Comp. Neurol. 424, 142–152 (2000).
Sadek, A. R., Magill, P. J. & Bolam, J. P. A single-cell analysis of intrinsic connectivity in the rat globus pallidus. J. Neurosci. 27, 6352–6362 (2007).
Bugaysen, J., Bar-Gad, I. & Korngreen, A. Continuous modulation of action potential firing by a unitary GABAergic connection in the globus pallidus in vitro. J. Neurosci. 33, 12805–12809 (2013).
Fujiyama, F. et al. A single-neuron tracing study of arkypallidal and prototypic neurons in healthy rats. Brain Struct. Funct. 221, 4733–4740 (2016).
Parent, A. et al. Organization of the basal ganglia: the importance of axonal collateralization. Trends Neurosci. 23, S20–S27 (2000).
Koshimizu, Y., Fujiyama, F., Nakamura, K. C., Furuta, T. & Kaneko, T. Quantitative analysis of axon bouton distribution of subthalamic nucleus neurons in the rat by single neuron visualization with a viral vector. J. Comp. Neurol. 521, 2125–2146 (2013).
Xiao, C. et al. Nicotinic receptor subtype-selective circuit patterns in the subthalamic nucleus. J. Neurosci. 35, 3734–3746 (2015).
Karube, F., Takahashi, S., Kobayashi, K. & Fujiyama, F. Motor cortex can directly drive the globus pallidus neurons in a projection neuron type-dependent manner in the rat. eLife 8, e49511 (2019).
Garcia, A. F., Crummy, E. A., Webb, I. G., Nooney, M. N. & Ferguson, S. M. Distinct populations of cortical pyramidal neurons mediate drug reward and aversion. Nat. Commun. 12, 182 (2021).
BRAIN Initiative Cell Census Network (BICCN). A multimodal cell census and atlas of the mammalian primary motor cortex. Nature 598, 86–102 (2021).
Muñoz-Castañeda, R. et al. Cellular anatomy of the mouse primary motor cortex. Nature 598, 159–166 (2021).
Parent, M. & Parent, A. Single-axon tracing and three-dimensional reconstruction of centre median–parafascicular thalamic neurons in primates. J. Comp. Neurol. 481, 127–144 (2005).
Yasukawa, T., Kita, T., Xue, Y. & Kita, H. Rat intralaminar thalamic nuclei projections to the globus pallidus: a biotinylated dextran amine anterograde tracing study. J. Comp. Neurol. 471, 153–167 (2004).
Kita, H., Nambu, A., Kaneda, K., Tachibana, Y. & Takada, M. Role of ionotropic glutamatergic and GABAergic inputs on the firing activity of neurons in the external pallidum in awake monkeys. J. Neurophysiol. 92, 3069–3084 (2004).
Jan, C. et al. Dopaminergic innervation of the pallidum in the normal state, in MPTP-treated monkeys and in parkinsonian patients. Eur. J. Neurosci. 12, 4525–4535 (2000).
Prensa, L., Cossette, M. & Parent, A. Dopaminergic innervation of human basal ganglia. J. Chem. Neuroanat. 20, 207–213 (2000).
Prensa, L. & Parent, A. The nigrostriatal pathway in the rat: a single-axon study of the relationship between dorsal and ventral tier nigral neurons and the striosome/matrix striatal compartments. J. Neurosci. 21, 7247–7260 (2001).
Debeir, T. et al. Effect of intrastriatal 6-OHDA lesion on dopaminergic innervation of the rat cortex and globus pallidus. Exp. Neurol. 193, 444–454 (2005).
Anaya-Martinez, V., Martinez-Marcos, A., Martinez-Fong, D., Aceves, J. & Erlij, D. Substantia nigra compacta neurons that innervate the reticular thalamic nucleus in the rat also project to striatum or globus pallidus: implications for abnormal motor behavior. Neuroscience 143, 477–486 (2006).
Smith, Y. & Villalba, R. Striatal and extrastriatal dopamine in the basal ganglia: an overview of its anatomical organization in normal and parkinsonian brains. Mov. Disord. 23, S534–S547 (2008).
Dopeso-Reyes, I. G. et al. Calbindin content and differential vulnerability of midbrain efferent dopaminergic neurons in macaques. Front. Neuroanat. 8, 146 (2014).
Aransay, A., Rodríguez-López, C., García-Amado, M., Clascá, F. & Prensa, L. Long-range projection neurons of the mouse ventral tegmental area: a single-cell axon tracing analysis. Front. Neuroanat. 9, 59 (2015).
Giovanniello, J. et al. A central amygdala–globus pallidus circuit conveys unconditioned stimulus-related information and controls fear learning. J. Neurosci. 40, 9043–9054 (2020).
Sztainberg, Y., Kuperman, Y., Justice, N. & Chen, A. An anxiolytic role for CRF receptor type 1 in the globus pallidus. J. Neurosci. 31, 17416–17424 (2011).
Pollak Dorocic, I. et al. A whole-brain atlas of inputs to serotonergic neurons of the dorsal and median raphe nuclei. Neuron 83, 663–678 (2014).
Stephenson-Jones, M. et al. A basal ganglia circuit for evaluating action outcomes. Nature 539, 289–293 (2016).
Qiu, M. H., Chen, M. C., Wu, J., Nelson, D. & Lu, J. Deep brain stimulation in the globus pallidus externa promotes sleep. Neuroscience 322, 115–120 (2016).
Wessel, J. R. & Aron, A. R. On the globality of motor suppression: unexpected events and their influence on behavior and cognition. Neuron 93, 259–280 (2017).
Fife, K. H. et al. Causal role for the subthalamic nucleus in interrupting behavior. eLife 6, e27689 (2017).
Schmidt, R., Leventhal, D. K., Mallet, N., Chen, F. & Berke, J. D. Canceling actions involves a race between basal ganglia pathways. Nat. Neurosci. 16, 1118–1124 (2013).
Hamani, C., Saint-Cyr, J. A., Fraser, J., Kaplitt, M. & Lozano, A. M. The subthalamic nucleus in the context of movement disorders. Brain 127, 4–20 (2004).
Aron, A. R. et al. Converging evidence for a fronto–basal–ganglia network for inhibitory control of action and cognition. J. Neurosci. 27, 11860–11864 (2007).
Aron, A. R. & Poldrack, R. A. Cortical and subcortical contributions to Stop signal response inhibition: role of the subthalamic nucleus. J. Neurosci. 26, 2424–2433 (2006).
Eagle, D. M. et al. Stop-signal reaction-time task performance: role of prefrontal cortex and subthalamic nucleus. Cereb. Cortex 18, 178–188 (2008).
Schweizer, N. et al. Limiting glutamate transmission in a Vglut2-expressing subpopulation of the subthalamic nucleus is sufficient to cause hyperlocomotion. Proc. Natl Acad. Sci. USA 111, 7837–7842 (2014).
Adam, E. M., Johns, T. & Sur, M. Dynamic control of visually guided locomotion through corticosubthalamic projections. Cell Rep. 40, 111139 (2022).
Sato, F., Lavallée, P., Lévesque, M. & Parent, A. Single-axon tracing study of neurons of the external segment of the globus pallidus in primate. J. Comp. Neurol. 417, 17–31 (2000).
Cui, G. et al. Concurrent activation of striatal direct and indirect pathways during action initiation. Nature 494, 238–242 (2013).
Markowitz, J. E. et al. The striatum organizes 3D behavior via moment-to-moment action selection. Cell 174, 44–58 (2018).
Maltese, M., March, J. R., Bashaw, A. G. & Tritsch, N. X. Dopamine differentially modulates the size of projection neuron ensembles in the intact and dopamine-depleted striatum. eLife 10, e68041 (2021).
Mallet, N. et al. Arkypallidal cells send a stop signal to striatum. Neuron 89, 308–316 (2016).
Beier, K. T. et al. Rabies screen reveals GPe control of cocaine-triggered plasticity. Nature 549, 345–350 (2017).
Watabe-Uchida, M., Zhu, L., Ogawa, S. K., Vamanrao, A. & Uchida, N. Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron 74, 858–873 (2012).
Lee, C. R., Abercrombie, E. D. & Tepper, J. M. Pallidal control of substantia nigra dopaminergic neuron firing pattern and its relation to extracellular neostriatal dopamine levels. Neuroscience 129, 481–489 (2004).
Cobb, W. S. & Abercrombie, E. D. Relative involvement of globus pallidus and subthalamic nucleus in the regulation of somatodendritic dopamine release in substantia nigra is dopamine-dependent. Neuroscience 119, 777–786 (2003).
Brazhnik, E., Shah, F. & Tepper, J. M. GABAergic afferents activate both GABAA and GABAB receptors in mouse substantia nigra dopaminergic neurons in vivo. J. Neurosci. 28, 10386–10398 (2008).
Evans, R. C. et al. Functional dissection of basal ganglia inhibitory inputs onto substantia nigra dopaminergic neurons. Cell Rep. 32, 108156 (2020).
Luo, L., Callaway, E. M. & Svoboda, K. Genetic dissection of neural circuits: a decade of progress. Neuron 98, 256–281 (2018).
Ahrlund-Richter, S. et al. A whole-brain atlas of monosynaptic input targeting four different cell types in the medial prefrontal cortex of the mouse. Nat. Neurosci. 22, 657–668 (2019).
Clayton, K. K. et al. Auditory corticothalamic neurons are recruited by motor preparatory inputs. Curr. Biol. 31, 310–321 (2020).
Vetrivelan, R., Qiu, M.-H., Chang, C. & Lu, J. Role of basal ganglia in sleep–wake regulation: neural circuitry and clinical significance. Front. Neuroanat. 4, 145 (2010).
Lazarus, M., Chen, J.-F., Urade, Y. & Huang, Z.-L. Role of the basal ganglia in the control of sleep and wakefulness. Curr. Opin. Neurobiol. 23, 780–785 (2013).
Dwi Wahyu, I., Chiken, S., Hasegawa, T., Sano, H. & Nambu, A. Abnormal cortico–basal ganglia neurotransmission in a mouse model of l-DOPA-induced dyskinesia. J. Neurosci. 41, 2668–2683 (2021).
Raz, A., Vaadia, E. & Bergman, H. Firing patterns and correlations of spontaneous discharge of pallidal neurons in the normal and the tremulous 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine vervet model of parkinsonism. J. Neurosci. 20, 8559–8571 (2000).
Magill, P. J., Bolam, J. P. & Bevan, M. D. Dopamine regulates the impact of the cerebral cortex on the subthalamic nucleus–globus pallidus network. Neuroscience 106, 313–330 (2001).
Chan, C. S. et al. HCN channelopathy in external globus pallidus neurons in models of Parkinson’s disease. Nat. Neurosci. 14, 85–92 (2011).
Leventhal, D. K. et al. Basal ganglia β oscillations accompany cue utilization. Neuron 73, 523–536 (2012).
Kuhn, A. A., Kupsch, A., Schneider, G. H. & Brown, P. Reduction in subthalamic 8–35 Hz oscillatory activity correlates with clinical improvement in Parkinson’s disease. Eur. J. Neurosci. 23, 1956–1960 (2006).
Gatev, P., Darbin, O. & Wichmann, T. Oscillations in the basal ganglia under normal conditions and in movement disorders. Mov. Disord. 21, 1566–1577 (2006).
Little, S. & Brown, P. The functional role of β oscillations in Parkinson’s disease. Parkinsonism Relat. Disord. 20, S44–S48 (2014).
Stein, E. & Bar-Gad, I. β oscillations in the cortico–basal ganglia loop during parkinsonism. Exp. Neurol. 245, 52–59 (2013).
de la Crompe, B. et al. The globus pallidus orchestrates abnormal network dynamics in a model of parkinsonism. Nat. Commun. 11, 1570 (2020).
Kovaleski, R. F. et al. Dysregulation of external globus pallidus–subthalamic nucleus network dynamics in parkinsonian mice during cortical slow-wave activity and activation. J. Physiol. 598, 1897–1927 (2020).
Starr, M. S., Starr, B. S. & Kaur, S. Stimulation of basal and l-DOPA-induced motor activity by glutamate antagonists in animal models of Parkinson’s disease. Neurosci. Biobehav. Rev. 21, 437–446 (1997).
Kelsey, J. E. et al. NMDA receptor antagonists ameliorate the stepping deficits produced by unilateral medial forebrain bundle injections of 6-OHDA in rats. Psychopharmacology 175, 179–188 (2004).
Liu, J. et al. Facilitation of GluN2C-containing NMDA receptors in the external globus pallidus increases firing of fast spiking neurons and improves motor function in a hemiparkinsonian mouse model. Neurobiol. Dis. 150, 105254 (2021).
Cui, Q. et al. Blunted mGluR activation disinhibits striatopallidal transmission in parkinsonian mice. Cell Rep. 17, 2431–2444 (2016).
Fan, K. Y., Baufreton, J., Surmeier, D. J., Chan, C. S. & Bevan, M. D. Proliferation of external globus pallidus–subthalamic nucleus synapses following degeneration of midbrain dopamine neurons. J. Neurosci. 32, 13718–13728 (2012).
Chu, H. Y., Atherton, J. F., Wokosin, D., Surmeier, D. J. & Bevan, M. D. Heterosynaptic regulation of external globus pallidus inputs to the subthalamic nucleus by the motor cortex. Neuron 85, 364–376 (2015).
Spix, T. A. et al. Population-specific neuromodulation prolongs therapeutic benefits of deep brain stimulation. Science 374, 201–206 (2021).
Starr, P. A., Kang, G. A., Heath, S., Shimamoto, S. & Turner, R. S. Pallidal neuronal discharge in Huntington’s disease: support for selective loss of striatal cells originating the indirect pathway. Exp. Neurol. 211, 227–233 (2008).
Temel, Y. et al. Motor and cognitive improvement by deep brain stimulation in a transgenic rat model of Huntington’s disease. Neurosci. Lett. 406, 138–141 (2006).
Beste, C. et al. Behavioral and neurophysiological evidence for the enhancement of cognitive control under dorsal pallidal deep brain stimulation in Huntington’s disease. Brain Struct. Funct. 220, 2441–2448 (2015).
Reiner, A., Dragatsis, I. & Dietrich, P. Genetics and neuropathology of Huntington’s disease. Int. Rev. Neurobiol. 98, 325–372 (2011).
Waldvogel, H. J., Kim, E. H., Tippett, L. J., Vonsattel, J.-P. G. & Faull, R. L. M. The neuropathology of Huntington’s disease. Curr. Top. Behav. Neurosci. 22, 33–80 (2015).
Deng, Y., Wang, H., Joni, M., Sekhri, R. & Reiner, A. Progression of basal ganglia pathology in heterozygous Q175 knock-in Huntington’s disease mice. J. Comp. Neurol. 529, 1327–1371 (2021).
Waldvogel, H. J. & Faull, R. L. M. The diversity of GABAA receptor subunit distribution in the normal and Huntington’s disease human brain. Adv. Pharmacol. 73, 223–264 (2015).
Perez-Rosello, T. et al. Enhanced striatopallidal γ-aminobutyric acid (GABA)A receptor transmission in mouse models of Huntington’s disease. Mov. Disord. 34, 684–696 (2019).
Barry, J., Akopian, G., Cepeda, C. & Levine, M. S. Striatal direct and indirect pathway output structures are differentially altered in mouse models of Huntington’s disease. J. Neurosci. 38, 4678–4694 (2018).
Plotkin, J. L. et al. Impaired TrkB receptor signaling underlies corticostriatal dysfunction in Huntington’s disease. Neuron 83, 178–188 (2014).
Sebastianutto, I., Cenci, M. A. & Fieblinger, T. Alterations of striatal indirect pathway neurons precede motor deficits in two mouse models of Huntington’s disease. Neurobiol. Dis. 105, 117–131 (2017).
Carrillo-Reid, L. et al. Mutant huntingtin enhances activation of dendritic Kv4 K+ channels in striatal spiny projection neurons. eLife 8, e40818 (2019).
Callahan, J. W., Wokosin, D. L. & Bevan, M. D. Dysregulation of the basal ganglia indirect pathway in early symptomatic Q175 Huntington’s disease mice. J. Neurosci. 42, 2080–2102 (2022).
Breakefield, X. O. et al. The pathophysiological basis of dystonias. Nat. Rev. Neurosci. 9, 222–234 (2008).
Tanabe, L. M., Kim, C. E., Alagem, N. & Dauer, W. T. Primary dystonia: molecules and mechanisms. Nat. Rev. Neurol. 5, 598–609 (2009).
Starr, P. A. et al. Spontaneous pallidal neuronal activity in human dystonia: comparison with Parkinson’s disease and normal macaque. J. Neurophysiol. 93, 3165–3176 (2005).
Chiken, S., Shashidharan, P. & Nambu, A. Cortically evoked long-lasting inhibition of pallidal neurons in a transgenic mouse model of dystonia. J. Neurosci. 28, 13967–13977 (2008).
Baron, M. S., Chaniary, K. D., Rice, A. C. & Shapiro, S. M. Multi-neuronal recordings in the basal ganglia in normal and dystonic rats. Front. Syst. Neurosci. 5, 67 (2011).
Nambu, A. et al. Reduced pallidal output causes dystonia. Front. Syst. Neurosci. 5, 89 (2011).
Nishibayashi, H. et al. Cortically evoked responses of human pallidal neurons recorded during stereotactic neurosurgery. Mov. Disord. 26, 469–476 (2011).
Sciamanna, G. et al. Optogenetic activation of striatopallidal neurons reveals altered HCN gating in DYT1 dystonia. Cell Rep. 31, 107644 (2020).
Assaf, F. & Schiller, Y. A chemogenetic approach for treating experimental Parkinson’s disease. Mov. Disord. 34, 469–479 (2019).
Mallet, N. et al. Parkinsonian β oscillations in the external globus pallidus and their relationship with subthalamic nucleus activity. J. Neurosci. 28, 14245–14258 (2008).
Acknowledgements
We thank past and current members of the Chan laboratory for their creativity and dedication to our understanding of the pallidum. This work was supported by NIH NS069777 (C.S.C.), MH112768 (C.S.C.), NS097901 (C.S.C.), MH109466 (C.S.C.), NS088528 (C.S.C.), AG020506 (A.P.) and a Parkinson’s Foundation postdoctoral fellowship (PF-PRF-837511 to C.D.C.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Neuroscience thanks Genevieve Konopka and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Courtney, C.D., Pamukcu, A. & Chan, C.S. Cell and circuit complexity of the external globus pallidus. Nat Neurosci 26, 1147–1159 (2023). https://doi.org/10.1038/s41593-023-01368-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-023-01368-7
This article is cited by
-
A non-canonical striatopallidal Go pathway that supports motor control
Nature Communications (2023)