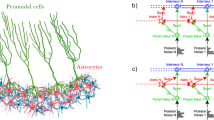
Abstract
The participation of astrocytes in brain computation was hypothesized in 1992, coinciding with the discovery that these cells display a form of intracellular Ca2+ signaling sensitive to neuroactive molecules. This finding fostered conceptual leaps crystalized around the idea that astrocytes, once thought to be passive, participate actively in brain signaling and outputs. A multitude of disparate roles of astrocytes has since emerged, but their meaningful integration has been muddied by the lack of consensus and models of how we conceive the functional position of these cells in brain circuitry. In this Perspective, we propose an intuitive, data-driven and transferable conceptual framework we coin ‘contextual guidance’. It describes astrocytes as ‘contextual gates’ that shape neural circuitry in an adaptive, state-dependent fashion. This paradigm provides fresh perspectives on principles of astrocyte signaling and its relevance to brain function, which could spur new experimental avenues, including in computational space.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author upon request.
Code availability
Codes and mathematical algorithms used in Fig. 4 will be made available upon publication.
References
Cornell-Bell, A. H., Finkbeiner, S. M., Cooper, M. S. & Smith, S. J. Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science 247, 470–473 (1990).
Smith, S. J. Do astrocytes process neural information? Prog. Brain Res. 94, 119–136 (1992).
Verkhratsky, A. & Nedergaard, M. Physiology of astroglia. Physiol. Rev. 98, 239–389 (2018).
Pellerin, L. & Magistretti, P. J. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc. Natl Acad. Sci. USA 91, 10625–10629 (1994).
Smith, S. J. Neural signalling. Neuromodulatory astrocytes. Curr. Biol. 4, 807–810 (1994).
Araque, A., Parpura, V., Sanzgiri, R. P. & Haydon, P. G. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 22, 208–215 (1999).
Nagai, J. et al. Behaviorally consequential astrocytic regulation of neural circuits. Neuron 109, 576–596 (2021).
Aten, S. et al. Ultrastructural view of astrocyte arborization, astrocyte-astrocyte and astrocyte-synapse contacts, intracellular vesicle-like structures, and mitochondrial network. Prog. Neurobiol. 213, 102264 (2022).
Bushong, E. A., Martone, M. E., Jones, Y. Z. & Ellisman, M. H. Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J. Neurosci. 22, 183–192 (2002).
Salmon, C. K. et al. Organizing principles of astrocytic nanoarchitecture in the mouse cerebral cortex. Curr. Biol. 33, 957–972 (2023).
Hösli, L. et al. Direct vascular contact is a hallmark of cerebral astrocytes. Cell Rep. 39, 110599 (2022).
Endo, F. et al. Molecular basis of astrocyte diversity and morphology across the CNS in health and disease. Science 378, eadc9020 (2022).
Semyanov, A. & Verkhratsky, A. Astrocytic processes: from tripartite synapses to the active milieu. Trends Neurosci. 44, 781–792 (2021).
Díaz-Castro, B., Robel, S. & Mishra, A. Astrocyte endfeet in brain function and pathology: open questions. Annu. Rev. Neurosci. 46, 101–121 (2023).
Gourine, A. V. et al. Astrocytes control breathing through pH-dependent release of ATP. Science 329, 571–575 (2010).
Xie, L. et al. Sleep drives metabolite clearance from the adult brain. Science 342, 373–377 (2013).
Wang, F. et al. Astrocytes modulate neural network activity by Ca²+-dependent uptake of extracellular K+. Sci. Signal. 5, ra26 (2012).
Henneberger, C., Papouin, T., Oliet, S. H. & Rusakov, D. A. Long-term potentiation depends on release of d-serine from astrocytes. Nature 463, 232–236 (2010).
Ding, F. et al. Changes in the composition of brain interstitial ions control the sleep-wake cycle. Science 352, 550–555 (2016).
Tønnesen, J., Inavalli, V. V. G. K. & Nägerl, U. V. Super-resolution imaging of the extracellular space in living brain tissue. Cell 172, 1108–1121 (2018).
Murphy-Royal, C. et al. Stress gates an astrocytic energy reservoir to impair synaptic plasticity. Nat. Commun. 11, 2014 (2020).
Kasymov, V. et al. Differential sensitivity of brainstem versus cortical astrocytes to changes in pH reveals functional regional specialization of astroglia. J. Neurosci. 33, 435–441 (2013).
Khakh, B. S. & Deneen, B. The emerging nature of astrocyte diversity. Annu. Rev. Neurosci. 42, 187–207 (2019).
Lanjakornsiripan, D. et al. Layer-specific morphological and molecular differences in neocortical astrocytes and their dependence on neuronal layers. Nat. Commun. 9, 1623 (2018).
Bayraktar, O. A. et al. Astrocyte layers in the mammalian cerebral cortex revealed by a single-cell in situ transcriptomic map. Nat. Neurosci. 23, 500–509 (2020).
Batiuk, M. Y. et al. Identification of region-specific astrocyte subtypes at single cell resolution. Nat. Commun. 11, 1220 (2020).
Chai, H. et al. Neural circuit-specialized astrocytes: transcriptomic, proteomic, morphological, and functional evidence. Neuron 95, 531–549 (2017).
Rusakov, D. A., Bard, L., Stewart, M. G. & Henneberger, C. Diversity of astroglial functions alludes to subcellular specialisation. Trends Neurosci. 37, 228–242 (2014).
Papouin, T., Dunphy, J. M., Tolman, M., Dineley, K. T. & Haydon, P. G. Septal cholinergic neuromodulation tunes the astrocyte-dependent gating of hippocampal NMDA receptors to wakefulness. Neuron 94, 840–854 (2017).
Ameroso, D. et al. Astrocytic BDNF signaling within the ventromedial hypothalamus regulates energy homeostasis. Nat. Metab. 4, 627–643 (2022).
Zuend, M. et al. Arousal-induced cortical activity triggers lactate release from astrocytes. Nat. Metab. 2, 179–191 (2020).
Tran, C. H. T., Peringod, G. & Gordon, G. R. Astrocytes integrate behavioral state and vascular signals during functional hyperemia. Neuron 100, 1133–1148 (2018).
Morquette, P. et al. An astrocyte-dependent mechanism for neuronal rhythmogenesis. Nat. Neurosci. 18, 844–854 (2015).
Lee, J. H. et al. Astrocytes phagocytose adult hippocampal synapses for circuit homeostasis. Nature 590, 612–617 (2021).
Panatier, A. et al. Glia-derived d-serine controls NMDA receptor activity and synaptic memory. Cell 125, 775–784 (2006).
Paukert, M. et al. Norepinephrine controls astroglial responsiveness to local circuit activity. Neuron 82, 1263–1270 (2014).
Bojarskaite, L. et al. Astrocytic Ca2+ signaling is reduced during sleep and is involved in the regulation of slow wave sleep. Nat. Commun. 11, 3240 (2020).
Burda, J. E. et al. Divergent transcriptional regulation of astrocyte reactivity across disorders. Nature 606, 557–564 (2022).
Heiman, M. G. & Shaham, S. Ancestral roles of glia suggested by the nervous system of Caenorhabditis elegans. Neuron Glia Biol. 3, 55–61 (2007).
Stobart, J. L. et al. Cortical circuit activity evokes rapid astrocyte calcium signals on a similar timescale to neurons. Neuron 98, 726–735 (2018).
Vardjan, N., Parpura, V. & Zorec, R. Loose excitation-secretion coupling in astrocytes. Glia 64, 655–667 (2016).
Bindocci, E. et al. Three-dimensional Ca2+ imaging advances understanding of astrocyte biology. Science 356, eaai8185 (2017).
Wu, Y. W. et al. Spatiotemporal calcium dynamics in single astrocytes and its modulation by neuronal activity. Cell Calcium 55, 119–129 (2014).
Lines, J., Martin, E. D., Kofuji, P., Aguilar, J. & Araque, A. Astrocytes modulate sensory-evoked neuronal network activity. Nat. Commun. 11, 3689 (2020).
Robin, L. M. et al. Astroglial CB1 receptors determine synaptic d-serine availability to enable recognition memory. Neuron 98, 935–944 (2018).
Martín, R., Bajo-Grañeras, R., Moratalla, R., Perea, G. & Araque, A. Circuit-specific signaling in astrocyte–neuron networks in basal ganglia pathways. Science 349, 730–734 (2015).
Takano, T. et al. Chemico-genetic discovery of astrocytic control of inhibition in vivo. Nature 588, 296–302 (2020).
Matos, M. et al. Astrocytes detect and upregulate transmission at inhibitory synapses of somatostatin interneurons onto pyramidal cells. Nat. Commun. 9, 4254 (2018).
Deemyad, T., Lüthi, J. & Spruston, N. Astrocytes integrate and drive action potential firing in inhibitory subnetworks. Nat. Commun. 9, 4336 (2018).
Stefanelli, T., Bertollini, C., Lüscher, C., Muller, D. & Mendez, P. Hippocampal somatostatin interneurons control the size of neuronal memory ensembles. Neuron 89, 1074–1085 (2016).
Mariotti, L. et al. Interneuron-specific signaling evokes distinctive somatostatin-mediated responses in adult cortical astrocytes. Nat. Commun. 9, 82 (2018).
Kohro, Y. et al. Spinal astrocytes in superficial laminae gate brainstem descending control of mechanosensory hypersensitivity. Nat. Neurosci. 23, 1376–1387 (2020).
Pankratov, Y. & Lalo, U. Role for astroglial α1-adrenoreceptors in gliotransmission and control of synaptic plasticity in the neocortex. Front. Cell. Neurosci. 9, 230 (2015).
Wahis, J. et al. Astrocytes mediate the effect of oxytocin in the central amygdala on neuronal activity and affective states in rodents. Nat. Neurosci. 24, 529–541 (2021).
Takata, N. et al. Astrocyte calcium signaling transforms cholinergic modulation to cortical plasticity in vivo. J. Neurosci. 31, 18155–18165 (2011).
Poskanzer, K. E. & Molofsky, A. V. Dynamism of an astrocyte in vivo: perspectives on identity and function. Annu. Rev. Physiol. 80, 143–157 (2018).
Gau, Y. A. et al. Multicore fiber optic imaging reveals that astrocyte calcium activity in the cerebral cortex is modulated by internal motivational state. Preprint at bioRxiv https://doi.org/10.1101/2023.05.18.541390 (2023).
Suthard, R. L. et al. Basolateral amygdala astrocytes are engaged by the acquisition and expression of a contextual fear memory. J. Neurosci. 43, 4997–5013 (2022).
Adedipe, I. I. et al. Astrocyte glucocorticoid signaling mediates cognitive impairment induced by early-life stress. Preprint at bioRxiv https://doi.org/10.1101/2022.12.11.519598 (2022).
Sapkota, D. et al. Activity-dependent translation dynamically alters the proteome of the perisynaptic astrocyte process. Cell Rep. 41, 111474 (2022).
Di Castro, M. A. & Volterra, A. Astrocyte control of the entorhinal cortex-dentate gyrus circuit: relevance to cognitive processing and impairment in pathology. Glia 70, 1536–1553 (2022).
Oe, Y. et al. Distinct temporal integration of noradrenaline signaling by astrocytic second messengers during vigilance. Nat. Commun. 11, 471 (2020).
Armbruster, M. et al. Neuronal activity drives pathway-specific depolarization of peripheral astrocyte processes. Nat. Neurosci. 25, 607–616 (2022).
Giaume, C., Koulakoff, A., Roux, L., Holcman, D. & Rouach, N. Astroglial networks: a step further in neuroglial and gliovascular interactions. Nat. Rev. Neurosci. 11, 87–99 (2010).
Longden, T. A. et al. Local IP3 receptor-mediated Ca2+ signals compound to direct blood flow in brain capillaries. Sci. Adv. 7, eabh0101 (2021).
García-Cáceres, C. et al. Astrocytic insulin signaling couples brain glucose uptake with nutrient availability. Cell 166, 867–880 (2016).
Kim, J. G. et al. Leptin signaling in astrocytes regulates hypothalamic neuronal circuits and feeding. Nat. Neurosci. 17, 908–910 (2014).
Fuente-Martín, E. et al. Ghrelin regulates glucose and glutamate transporters in hypothalamic astrocytes. Sci. Rep. 6, 23673 (2016).
Crosby, K. M. et al. Cholecystokinin switches the plasticity of GABA synapses in the dorsomedial hypothalamus via astrocytic ATP release. J. Neurosci. 38, 8515–8525 (2018).
Zerbi, V. et al. Rapid reconfiguration of the functional connectome after chemogenetic locus coeruleus activation. Neuron 103, 702–718 (2019).
Pacholko, A. G., Wotton, C. A. & Bekar, L. K. Astrocytes—the ultimate effectors of long-range neuromodulatory networks? Front. Cell. Neurosci. 14, 581075 (2020).
Hirase, H., Iwai, Y., Takata, N., Shinohara, Y. & Mishima, T. Volume transmission signalling via astrocytes. Philos. Trans. R. Soc. Lond. B Biol. Sci. 369, 20130604 (2014).
Rurak, G. M. et al. Sex differences in developmental patterns of neocortical astroglia: a mouse translatome database. Cell Rep. 38, 110310 (2022).
Richards, B., Tsao, D. & Zador, A. The application of artificial intelligence to biology and neuroscience. Cell 185, 2640–2643 (2022).
Parisi, G. I., Kemker, R., Part, J. L., Kanan, C. & Wermter, S. Continual lifelong learning with neural networks: a review. Neural Netw. 113, 54–71 (2019).
Yu, Y., Si, X., Hu, C. & Zhang, J. A review of recurrent neural networks: LSTM cells and network architectures. Neural Comput. 31, 1235–1270 (2019).
Corkrum, M. et al. Dopamine-evoked synaptic regulation in the nucleus accumbens requires astrocyte activity. Neuron 105, 1036–1047 (2020).
Mu, Y. et al. Glia accumulate evidence that actions are futile and suppress unsuccessful behavior. Cell 178, 27–43 (2019).
Acknowledgements
C.M.-R. was supported by a Canadian Institutes of Health Research Project Grant (478629), an NSERC Discovery Grant (RGPIN-2021-03211), Fonds de Recherche du Québec – Santé (296562 & 309889) and the Brain & Behavior Research Foundation (NARSAD Young Investigator Award 28589). S.C. was supported by the Department of Defense (DoD; W911NF-21-1-0312) and the National Science Foundation (1653589). T.P. was supported by the National Institutes of Health (1R01MH127163-01), the DoD (W911NF-21-1-0312), the Brain & Behavior Research Foundation (NARSAD Young Investigator Award 28616), the Whitehall Foundation (2020-08-35) and the McDonnell Center for Cellular and Molecular Neurobiology Award (22-3930-26275U). The authors thank J. Dunphy for critical feedback. We apologize that the work of many of our colleagues and peers could not be cited owing to strict space limitations and the abundance of papers published during the publication process. Figure 4 was created with BioRender.com.
Author information
Authors and Affiliations
Contributions
T.P. wrote the first drafts and created the figures, box and supplementary tables. C.M.-R. and S.C. contributed to sections along lines of expertise. C.M.-R. expanded and revised the manuscript. S.C. generated Fig. 4. T.P. assembled comments from all authors, wrote the final version and performed revisions.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Neuroscience thanks Inbal Goshen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables 1 and 2
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Murphy-Royal, C., Ching, S. & Papouin, T. A conceptual framework for astrocyte function. Nat Neurosci 26, 1848–1856 (2023). https://doi.org/10.1038/s41593-023-01448-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-023-01448-8