Abstract
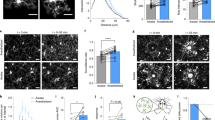
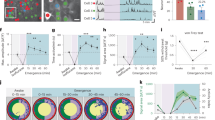
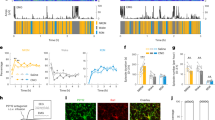
Microglia dynamically survey the brain parenchyma. Microglial processes interact with neuronal elements; however, what role neuronal network activity plays in regulating microglial dynamics is not entirely clear. Most studies of microglial dynamics use either slice preparations or in vivo imaging in anesthetized mice. Here we demonstrate that microglia in awake mice have a relatively reduced process area and surveillance territory and that reduced neuronal activity under general anesthesia increases microglial process velocity, extension and territory surveillance. Similarly, reductions in local neuronal activity through sensory deprivation or optogenetic inhibition increase microglial process surveillance. Using pharmacological and chemogenetic approaches, we demonstrate that reduced norepinephrine signaling is necessary for these increases in microglial process surveillance. These findings indicate that under basal physiological conditions, noradrenergic tone in awake mice suppresses microglial process surveillance. Our results emphasize the importance of awake imaging for studying microglia–neuron interactions and demonstrate how neuronal activity influences microglial process dynamics.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
Source data for all graphs are available from the corresponding author upon request.
References
Ransohoff, R. M. & Perry, V. H. Microglial physiology: unique stimuli, specialized responses. Annu. Rev. Immunol.27, 119–145 (2009).
Wolf, S. A., Boddeke, H. W. & Kettenmann, H. Microglia in physiology and disease. Annu. Rev. Physiol.79, 619–643 (2017).
Kettenmann, H., Hanisch, U. K., Noda, M. & Verkhratsky, A. Physiology of microglia. Physiol. Rev.91, 461–553 (2011).
Wu, Y., Dissing-Olesen, L., MacVicar, B. A. & Stevens, B. Microglia: dynamic mediators of synapse development and plasticity. Trends Immunol.36, 605–613 (2015).
Eyo, U. B. & Wu, L. J. Bi-directional microglia–neuron communication in the healthy brain. Neural Plast.2013, 456857 (2013).
Salter, M. W. & Stevens, B. Microglia emerge as central players in brain disease. Nat. Med.23, 1018–1027 (2017).
Miyamoto, A. et al. Microglia contact induces synapse formation in developing somatosensory cortex. Nat. Commun.7, 12540 (2016).
Weinhard, L. et al. Microglia remodel synapses by presynaptic trogocytosis and spine head filopodia induction. Nat. Commun.9, 1228 (2018).
Tremblay, M. E., Lowery, R. L. & Majewska, A. K. Microglial interactions with synapses are modulated by visual experience. PLoS Biol.8, e1000527 (2010).
Schafer, D. et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron74, 691–705 (2012).
Parkhurst, C. N. et al. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell155, 1596–1609 (2013).
Akiyoshi, R. et al. Microglia enhance synapse activity to promote local network synchronization. eNeuro5, ENEURO.0088-18.2018 (2018).
Nimmerjahn, A., Kirchhoff, F. & Helmchen, F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science308, 1314–1318 (2005).
Davalos, D. et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci.8, 752–758 (2005).
Eyo, U. B. et al. P2Y12R-dependent translocation mechanisms gate the changing microglial landscape. Cell Rep.23, 959–966 (2018).
Haynes, S. E. et al. The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat. Neurosci.9, 1512–1519 (2006).
Wu, L. J., Vadakkan, K. I. & Zhuo, M. ATP-induced chemotaxis of microglial processes requires P2Y receptor-activated initiation of outward potassium currents. Glia55, 810–821 (2007).
Wake, H., Moorhouse, A. J., Jinno, S., Kohsaka, S. & Nabekura, J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J. Neurosci.29, 3974–3980 (2009).
Eyo, U. B. et al. Regulation of physical microglia–neuron interactions by fractalkine signaling after status epilepticus. eNeuro3, ENEURO.0209-16.2016 (2017).
Eyo, U. B. et al. Neuronal hyperactivity recruits microglial processes via neuronal NMDA receptors and microglial P2Y12 receptors after status epilepticus. J. Neurosci.34, 10528–10540 (2014).
Madry, C. et al. Microglial ramification, surveillance, and interleukin-1beta release are regulated by the two-pore domain K(+) channel THIK-1. Neuron97, 299–312 e296 (2018).
Franks, N. P. General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat. Rev. Neurosci.9, 370–386 (2008).
Petersen, C. C. The functional organization of the barrel cortex. Neuron56, 339–355 (2007).
Zhao, S. et al. Cell type-specific channelrhodopsin-2 transgenic mice for optogenetic dissection of neural circuitry function. Nat. Methods8, 745–752 (2011).
Eyo, U. B., Murugan, M. & Wu, L. J. Microglia–neuron communication in epilepsy. Glia65, 5–18 (2017).
Dissing-Olesen, L. et al. Activation of neuronal NMDA receptors triggers transient ATP-mediated microglial process outgrowth. J. Neurosci.34, 10511–10527 (2014).
Liang, K. J. et al. Regulation of dynamic behavior of retinal microglia by CX3CR1 signaling. Invest. Ophthalmol. Vis. Sci.50, 4444–4451 (2009).
Wu, L. J. & Zhuo, M. Resting microglial motility is independent of synaptic plasticity in mammalian brain. J. Neurophysiol.99, 2026–2032 (2008).
Muller, C. P. et al. The in vivo neurochemistry of the brain during general anesthesia. J. Neurochem.119, 419–446 (2011).
Zhang, Y. et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci.34, 11929–11947 (2014).
Gyoneva, S. & Traynelis, S. F. Norepinephrine modulates the motility of resting and activated microglia via different adrenergic receptors. J. Biol. Chem.288, 15291–15302 (2013).
Schwarz, L. A. & Luo, L. Organization of the locus coeruleus–norepinephrine system. Curr. Biol.25, R1051–R1056 (2015).
Sipe, G. O. et al. Microglial P2Y12 is necessary for synaptic plasticity in mouse visual cortex. Nat. Commun.7, 10905 (2016).
Berridge, C. W. & Waterhouse, B. D. The locus coeruleus–noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res. Brain Res. Rev.42, 33–84 (2003).
Audet, M. A., Doucet, G., Oleskevich, S. & Descarries, L. Quantified regional and laminar distribution of the noradrenaline innervation in the anterior half of the adult rat cerebral cortex. J. Comp. Neurol.274, 307–318 (1988).
O’Donnell, J., Zeppenfeld, D., McConnell, E., Pena, S. & Nedergaard, M. Norepinephrine: a neuromodulator that boosts the function of multiple cell types to optimize CNS performance. Neurochem. Res.37, 2496–2512 (2012).
Kohm, A. P. & Sanders, V. M. Norepinephrine and beta 2-adrenergic receptor stimulation regulate CD4+ T and B lymphocyte function in vitro and in vivo. Pharm. Rev.53, 487–525 (2001).
Orr, A. G., Orr, A. L., Li, X. J., Gross, R. E. & Traynelis, S. F. Adenosine A2A receptor mediates microglial process retraction. Nat. Neurosci.12, 872–878 (2009).
Bernier, L. P. et al. Nanoscale surveillance of the brain by microglia via cAMP-regulated filopodia. Cell Rep.27, 2895–2908.e2894 (2019).
Eyo, U. B. et al. Modulation of microglial process convergence toward neuronal dendrites by extracellular calcium. J. Neurosci.35, 2417–2422 (2015).
Zhao, X., Eyo, U. B., Murugan, M. & Wu, L. J. Microglial interactions with the neurovascular system in physiology and pathology. Dev. Neurobiol.78, 604–617 (2018).
Li, Y., Du, X. F., Liu, C. S., Wen, Z. L. & Du, J. L. Reciprocal regulation between resting microglial dynamics and neuronal activity in vivo. Dev. Cell23, 1189–1202 (2012).
Chan-Palay, V. & Asan, E. Alterations in catecholamine neurons of the locus coeruleus in senile dementia of the Alzheimer type and in Parkinson’s disease with and without dementia and depression. J. Comp. Neurol.287, 373–392 (1989).
Kvetnansky, R., Sabban, E. L. & Palkovits, M. Catecholaminergic systems in stress: structural and molecular genetic approaches. Physiol. Rev.89, 535–606 (2009).
Heneka, M. T. et al. Locus ceruleus controls Alzheimer’s disease pathology by modulating microglial functions through norepinephrine. Proc. Natl Acad. Sci. USA107, 6058–6063 (2010).
Xu, H., Rajsombath, M. M., Weikop, P. & Selkoe, D. J. Enriched environment enhances beta-adrenergic signaling to prevent microglia inflammation by amyloid-beta. EMBO Mol. Med.10, e8931 (2018).
Hong, S. et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science352, 712–716 (2016).
Mitchell, H. A. & Weinshenker, D. Good night and good luck: norepinephrine in sleep pharmacology. Biochem. Pharm.79, 801–809 (2010).
Brown, E. N., Lydic, R. & Schiff, N. D. General anesthesia, sleep, and coma. N. Engl. J. Med.363, 2638–2650 (2010).
Jung, S. et al. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol. Cell. Biol.20, 4106–4114 (2000).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods9, 676–682 (2012).
Acknowledgements
This work is supported by the National Institutes of Health (R01NS088627, R21DE025689 and R01NS112144 to L.-J.W.) and by a postdoctoral fellowship from the Mayo Clinic Center for Multiple Sclerosis and Autoimmune Neurology (to T.C). The authors thank M. Mattson (National Institute on Aging) for critical reading of the paper and members of the Wu Lab at the Mayo Clinic for insightful discussions.
Author information
Authors and Affiliations
Contributions
Y.U.L., H.D. and L.-J.W. designed the studies. Y.U.L., A.D.U. and L.-J.W. wrote and revised the manuscript. Y.U.L. performed the electrophysiology experiments. Y.U.L., Y.Y., Y.L., U.B.E., T.C., J. Zheng, A.D.U., J .Zhu and D.B.B. performed animal surgery, image collection and data analyses. Y.U.L. and J. Zheng performed the in situ hybridization and immunofluorescence staining experiments.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–12.
Supplementary Video 1
Microglial process surveillance increases after anesthesia. Time-lapse imaging of microglia (50–64 µm in depth) in the somatosensory cortex before and after isoflurane anesthesia. Experiments were repeated three times independently with similar results.
Supplementary Video 2
Neuronal network activity in the somatosensory cortex immediately decreases after isoflurane anesthesia. Calcium activity in excitatory CamKII-positive soma (150 µm in depth, left) or dendrites (50 µm, right) before and after isoflurane induction. Experiments were repeated three times independently with similar results.
Supplementary Video 3
Neuronal network activity in the barrel cortex decreases after contralateral whisker trimming. Calcium activity in CamKII neuronal dendrites (50 µm in depth, right) of the contralateral barrel cortex before and after whisker trimming. Experiments were repeated three times independently with similar results.
Supplementary Video 4
Microglial process surveillance increases after contralateral whisker trimming. Time-lapse imaging of microglia (50–64 µm in depth) in the contralateral barrel cortex before and after whisker trimming. Experiments were repeated three times independently with similar results.
Supplementary Video 5
Intracerebral administration of muscimol (870 µM) reduces neuronal network activity. Calcium activity of CamKII neuronal somas (150 µm in depth) in the somatosensory cortex before and after muscimol administration. Experiments were repeated three times independently with similar results.
Supplementary Video 6
Microglial process surveillance increases after intracerebral administration of muscimol (870 µM). Time-lapse imaging of microglia (50–64 µm depth) in somatosensory cortex before and after muscimol administration. Experiments were repeated three times independently with similar results.
Supplementary Video 7
Microglial process surveillance increases after optogenetic stimulation of VGAT-positive inhibitory neurons. Time-lapse imaging of microglia (50–64 µm depth) in the somatosensory cortex before and after optogenetic stimulation (10 Hz, 1 ms pulse, 10 min of stimulation) of ChR2-expressing VGAT-positive interneurons. Experiments were repeated three times independently with similar results.
Supplementary Video 8
In vivo imaging dense noradrenergic neuronal projections from the locus coeruleus. Z-stack movie showing td-Tomato-labeled axons, which project from noradrenergic neurons in the LC. The video progresses from the pial surface down to a 100 µm in depth in the somatosensory cortex. Experiments were repeated three times independently with similar results.
Supplementary Video 9
Neuronal network activity in acute brain slices exposed to glutamate. Calcium activity in excitatory CamKII-positive soma in three cortical slices exposed to vehicle, 50 µM glutamate or 1 mM glutamate. Experiments were repeated three times independently with similar results.
Rights and permissions
About this article
Cite this article
Liu, Y.U., Ying, Y., Li, Y. et al. Neuronal network activity controls microglial process surveillance in awake mice via norepinephrine signaling. Nat Neurosci 22, 1771–1781 (2019). https://doi.org/10.1038/s41593-019-0511-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-019-0511-3
This article is cited by
-
APOE4 genotype and aging impair injury-induced microglial behavior in brain slices, including toward Aβ, through P2RY12
Molecular Neurodegeneration (2024)
-
Linking activity dyshomeostasis and sleep disturbances in Alzheimer disease
Nature Reviews Neuroscience (2024)
-
Microglia regulate sleep through calcium-dependent modulation of norepinephrine transmission
Nature Neuroscience (2024)
-
Lifelong absence of microglia alters hippocampal glutamatergic networks but not synapse and spine density
EMBO Reports (2024)
-
Microglia enhance post-anesthesia neuronal activity by shielding inhibitory synapses
Nature Neuroscience (2024)