Abstract
In the STEP-HFpEF trial, semaglutide improved symptoms, physical limitations and exercise function and reduced body weight in patients with obesity phenotype of heart failure and preserved ejection fraction (HFpEF). This prespecified analysis examined the effects of semaglutide on dual primary endpoints (change in Kansas City Cardiomyopathy Questionnaire-Clinical Summary Score (KCCQ-CSS) and body weight) and confirmatory secondary endpoints (change in 6-minute walk distance (6MWD), hierarchical composite (death, HF events, change in KCCQ-CSS and 6MWD) and change in C-reactive protein (CRP)) across obesity classes I–III (body mass index (BMI) 30.0–34.9 kg m−2, 35.0–39.9 kg m−2 and ≥40 kg m−2) and according to body weight reduction with semaglutide after 52 weeks. Semaglutide consistently improved all outcomes across obesity categories (P value for treatment effects × BMI interactions = not significant for all). In semaglutide-treated patients, improvements in KCCQ-CSS, 6MWD and CRP were greater with larger body weight reduction (for example, 6.4-point (95% confidence interval (CI): 4.1, 8.8) and 14.4-m (95% CI: 5.5, 23.3) improvements in KCCQ-CSS and 6MWD for each 10% body weight reduction). In participants with obesity phenotype of HFpEF, semaglutide improved symptoms, physical limitations and exercise function and reduced inflammation and body weight across obesity categories. In semaglutide-treated patients, the magnitude of benefit was directly related to the extent of weight loss. Collectively, these data support semaglutide-mediated weight loss as a key treatment strategy in patients with obesity phenotype of HFpEF. ClinicalTrials.gov identifier: NCT04788511.
Similar content being viewed by others
Main
The prevalence of heart failure with preserved ejection fraction (HFpEF) is increasing worldwide, and there are few effective treatments1,2. Approximately 60% of patients with HFpEF have the obesity phenotype3, which is a pathophysiologically distinct form of HFpEF characterized by greater symptom severity, poorer exercise capacity, more adverse hemodynamics and greater risk for HF hospitalization than those with HFpEF without obesity3,4,5,6,7,8,9,10. In the STEP-HFpEF trial, treatment with 2.4 mg of the glucagon-like peptide-1 receptor agonist semaglutide weekly produced substantial improvements in symptoms, physical limitations and exercise function and reduced inflammation and resulted in greater weight loss compared to placebo11,12.
However, it is not known if the observed effects of semaglutide in STEP-HFpEF vary by obesity class. Obesity is traditionally defined as body mass index (BMI) of 30 kg m−2 or greater, but, within this broad definition, there is substantial variation in the amount of excess adiposity. In the United States, approximately one-third of patients with the obesity phenotype of HFpEF have class III obesity, defined by BMI ≥40 kg m−2, whereas 40% of patients have class I obesity (BMI 30–34.9 kg m−2) (ref. 3). In cross-sectional studies, symptom severity, exercise limitations and hemodynamic abnormalities in the obesity phenotype of HFpEF worsen as BMI increases6,7,8, suggesting the possibility that beneficial effects from semaglutide could be mostly confined to individuals with HFpEF and very high BMI. Furthermore, it is unclear whether the magnitude of body weight reduction after treatment with semaglutide is related to the extent of clinical improvement in symptom severity, exercise function or systemic inflammation.
This prespecified analysis of STEP-HFpEF investigated the efficacy of semaglutide versus placebo in patients with HFpEF across the different classes of obesity, as it pertains to the primary endpoints (change in Kansas City Cardiomyopathy Questionnaire-Clinical Summary Score (KCCQ-CSS) and body weight) and confirmatory secondary endpoints (change in 6-minute walk distance (6MWD), hierarchical composite endpoint (comprising all-cause death, HF events, several thresholds of change in KCCQ-CSS and change in 6MWD ≥30 m) and change in C-reactive protein (CRP)), and it tested whether the degree of body weight reduction achieved on treatment with semaglutide was related to the improvements in the key trial endpoints.
Results
Patient characteristics
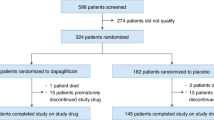
A total of 817 patients were screened, and, of this group, 529 fulfilled eligibility criteria and were enrolled and randomized between 19 March 2021 and 9 March 2022 (Extended Data Fig. 1). Among the 529 STEP-HFpEF participants, 263 and 266 were randomized to semaglutide versus placebo, respectively; the median BMI was 37.0 kg m−2 (33.7, 41.4) at baseline, 180 (34.0%) had class I obesity, 171 (32.3%) had class II obesity and 178 (33.7%) had class III obesity. Compared to patients who had less severe obesity, those with greater severity of obesity were more likely to be women and younger, with lower N-terminal pro-brain type natriuretic peptide (NTproBNP) levels but more severe impairments in HF symptoms, physical limitations and exercise function as reflected by lower KCCQ-CSS and 6MWD and higher New York Heart Association (NYHA) class and CRP levels (Table 1). No differences were observed in systolic blood pressure (SBP) or medical therapy for HF, except that patients with lower obesity class were more likely to be treated with sodium-glucose co-transporter-2 (SGLT2) inhibitors, and patients with higher obesity class were more likely to receive loop diuretics at higher dose. No differences were observed in the prevalence of hypertension, atrial fibrillation or sleep apnea by obesity class, but patients with increased severity of obesity were less likely to have history of coronary artery disease.
In regression analyses, increase in BMI was associated with lower KCCQ-CSS and 6MWD and higher CRP at the time of baseline assessment, after adjusting for age, sex, NYHA class, history of atrial fibrillation and history of coronary disease (Supplementary Table 1).
Treatment effects by baseline obesity class
As compared to placebo, treatment with semaglutide improved KCCQ-CSS and reduced body weight across all obesity categories (Fig. 1). Semaglutide also improved 6MWD, resulted in a greater number of wins versus placebo for the composite hierarchical endpoint and reduced systemic inflammation assessed by CRP in each obesity class, with no heterogeneity of treatment benefits (Fig. 2). These findings were observed in both the intention-to-treat analyses and the on-treatment analyses.
a,b, There was no evidence of heterogeneity in the effects of semaglutide compared to placebo on the dual primary endpoints of KCCQ-CSS (a) or body weight (b). Data are point estimates and 95% CIs. Analyses using the intention-to-treat principle employ an F-test for interaction and a Wald test for treatment effect within BMI subgroups, with 1,000 imputations using Rubin’s rule. Analyses using on-treatment data employ an F-test for interaction and a t-test for treatment effect within BMI subgroups. P values are two-sided. ETD, estimated treatment difference.
a,b, There was no evidence of heterogeneity in the effects of semaglutide compared to placebo on the confirmatory secondary endpoints of exercise function assessed by 6MWD (a), the hierarchical composite endpoint (b) or systemic inflammation assessed by CRP levels. Data are point estimates and 95% CIs. Analyses using the intention-to-treat principle employ an F-test (a,c) or Cohran’s Q-test (b) for interaction and a Wald test for treatment effect within BMI subgroups, with 1,000 imputations using Rubin’s rule. Analyses using on-treatment data employ an F-test for interaction and a t-test for treatment effect within BMI subgroups (a,c) or Cohran’s Q-test (b) for interaction and a Wald test for treatment effect within BMI subgroups. P values are two-sided. Other abbreviations as in Fig. 1.
Semaglutide effects and weight change
Among patients who were treated with semaglutide and had a recorded body weight at week 52, body weight reduction was <5% in 33 (13.4%) participants, 5–<10% in 51 (20.7%) participants, 10–<15% in 54 (22.0%) participants, 15–<20% in 50 (20.3%) participants and >20% in 58 (23.6%) participants. Increased degree of body weight reduction was associated with increased magnitude of improvements in KCCQ-CSS and 6MWD and reduction in CRP. These dose–response relationships between the amount of weight loss and treatment benefits were observed when body weight change was analyzed both as an ordinal (Fig. 3) and as a continuous variable, after adjusting for age, sex, NYHA class, history of atrial fibrillation and coronary artery disease, baseline CRP and NTproBNP at baseline (Table 2). Results were consistent between the intention-to-treat and on-treatment analyses, except for 6MWD where dose–response relationship was observed in the intention-to-treat, but not in the on-treatment, analysis (Extended Data Fig. 2).
a–c, Greater body weight reduction with semaglutide was associated with greater improvements in HF symptoms and physical limitations assessed by the KCCQ-CSS (a), exercise function assessed by the 6MWD (b) and greater reduction in systemic inflammation assessed by CRP levels (c). Data are point estimates and 95% CIs. Analyses use the intention-to-treat principle; tests for trend are based on an F-test. P values are two-sided. Abbreviations as in Figs. 1 and 2.
Based on the linear regression slopes (Table 2), each 10% reduction in body weight with semaglutide was associated with a 6.4-point (95% confidence interval (CI): 4.1, 8.8) increase in KCCQ-CSS, a 14.4-m (95% CI: 5.5, 23.3) increase in 6MWD and a 28% (95% CI: 16, 37) decrease in CRP, after adjusting for baseline age, sex, body weight, endpoint value, NYHA class, coronary artery disease, atrial fibrillation, CRP (log-transformed) and NTproBNP (log-transformed).
Safety and tolerability
There were fewer serious adverse events reported among participants randomized to semaglutide versus placebo within each obesity class, with no evidence of heterogeneity in safety or tolerability (Table 3). A similar (and small) number of patients discontinued study medication due to serious adverse events in the semaglutide and placebo groups. The number of deaths in the semaglutide and placebo groups, respectively, were 2 and 0 in obesity class I, 0 and 2 in obesity class II and 1 and 2 in obesity class III.
Discussion
In this prespecified analysis from the STEP-HFpEF trial, semaglutide as compared to placebo improved HF-related symptoms, physical limitations and exercise function and reduced body weight and inflammation across the spectrum of obesity categories. Furthermore, in patients treated with semaglutide, increased degree of weight loss was associated with increased magnitude of improvements in symptoms, physical limitations and inflammation, even after adjusting for relevant baseline characteristics that might influence treatment response, including age, sex and baseline body weight. These data demonstrate that the effects of semaglutide-induced weight loss are not restricted to individuals with very high BMI but apply across the entire spectrum of obesity. In addition, the relationships between the magnitude of weight reduction and clinical efficacy provide mechanistic evidence supporting the importance of weight reduction as an effective treatment for patients with the obesity phenotype of HFpEF.
Patients with the obesity phenotype of HFpEF display distinct pathophysiologic characteristics compared to patients with other phenotypes of HFpEF, including greater volume expansion, higher cardiac filling pressures, more severe right-sided HF and increases in epicardial fat that amplify external constraint on the heart6. It has been shown that patients with the obesity HFpEF phenotype are younger and have lower natriuretic peptide levels3,6,7 but present with higher NYHA class4,7, greater symptom severity, poorer exercise capacity7,8 and greater systemic inflammation than patients without obesity7. These relationships with obesity severity in patients with HFpEF were again observed in the present analysis, supporting the validity and generalizability of these data from the STEP-HFpEF trial.
Previous studies showed direct linear relationships between body weight and symptom severity, exercise limitation and hemodynamic abnormalities in patients with the obesity phenotype of HFpEF6,7,8. These relationships might support a hypothesis that only those individuals with HFpEF and the most severe obesity phenotypes would benefit from weight loss treatments. However, in this analysis, we observed similar treatment benefits of semaglutide for all primary and confirmatory secondary endpoints across the spectrum of obesity categories. These findings have important clinical implications, as approximately 40% of patients with the obesity phenotype of HFpEF have only mild obesity (class I), and the present analyses indicate that these patients benefit just as much as patients with more severe obesity3. The relationship observed between reductions in body weight and improvements in symptoms and physical limitations supports the hypothesis that the obesity phenotype of HFpEF is, in large part, a consequence of increased adiposity and its many sequelae, although there are also likely non-obesity-related contributors to the pathophysiology.
Weight loss in patients with obesity but no HF is associated with effects that would be expected to reduce symptom severity and improve exercise function in patients with HFpEF, including reversal of hypertrophic chamber remodeling, improvement in ventricular mechanics and reductions in hemodynamic congestion13,14. The present findings are supported by the results of the SECRET trial, which showed that non-pharmacologic body weight reduction achieved through caloric restriction improved exercise capacity in patients with the obesity phenotype of HFpEF15. The present study directly relates the degree of pharmacologically mediated weight loss with the magnitude of clinical benefits observed across the broad range of outcomes, including symptoms and physical limitations (KCCQ-CSS), exercise function (6MWD) and inflammation (CRP). These benefits are not simply ascribable to mechanical effects of body weight reduction, as the STEP-HFpEF trial also showed that semaglutide reduced NTproBNP levels compared to placebo, consistent with a direct benefit on hemodynamic congestion11.
The findings of this study should be considered in the context of several potential limitations. Most participants in STEP-HFpEF were White, and individuals with diabetes were excluded by design, which may affect the generalizability to non-White populations and people with diabetes. A separate, ongoing trial is evaluating the effects of semaglutide in people with the obesity phenotype of HFpEF and type 2 diabetes12. The STEP-HFpEF trial was designed to evaluate the effects of treatment on symptoms and physical limitations, exercise function and inflammation, along with body weight, and was, therefore, not powered to assess clinical endpoints such as HF hospitalizations. Power is reduced by focusing on obesity class subgroups as compared to the main analysis, but the findings were consistent across obesity categories with no evidence for heterogeneity of treatment effects. The 52-week duration of treatment was relatively short, and whether the observed effects might have persisted (or become more amplified) with longer evaluation is not known. Use of SGLT2 inhibitors was low in STEP-HFpEF, as patients with diabetes were excluded, and these agents were not yet approved for treatment of HFpEF during the trial conduct. Although semaglutide and SGLT2 inhibitors have complementary and non-overlapping mechanisms of action, the present study cannot determine whether background therapy with SGLT2 inhibitors might have influenced the treatment benefits observed, which is an important question for future trials. Further insight into the effects of semaglutide in patients who receive background SGLT2 inhibitors will be provided by the STEP-HFpEF DM trial, which includes a greater proportion (32%) of patients taking these agents12. BMI is a crude measure of adiposity that does not assess body composition or quantity of visceral fat, which has more deleterious effects in HFpEF6,16, limiting insight on the effect of semaglutide on visceral fat loss and its association with improvements in KCCQ-CSS and 6MWD outcomes.
In the STEP-HFpEF trial of participants with the obesity phenotype of HFpEF, semaglutide improved symptoms, physical limitations and exercise function and reduced inflammation and body weight across the spectrum of obesity categories. In semaglutide-treated patients, the magnitude of benefit was directly related to the extent of weight loss. Collectively, these data support semaglutide-mediated weight loss as a key treatment strategy in patients with the obesity phenotype of HFpEF.
Methods
Study design
STEP-HFpEF (NCT04788511) was a randomized, international, double-blind, placebo-controlled trial that examined the efficacy and safety of semaglutide 2.4 mg once weekly compared to placebo in patients with the obesity phenotype of HFpEF without diabetes11. The study design and the primary results were previously published11,12. Institutional review board ethics approval was obtained at each study site, and all patients provided informed consent to participate in the trial.
Study patients
Eligible participants were randomized 1:1 to semaglutide 2.4 mg subcutaneously or matching placebo once weekly in addition to standard of care for 52 weeks12. For all participants, frequent physical activity of moderate intensity (as tolerated in HFpEF) and limited consumption of salt, red meat, saturated or trans fats, sweets and sugar-sweetened beverages, with restricted calorie intake (goal, 500 kcal deficit per day) were recommended. Smoking cessation was supported, and alcohol consumption was recommended to be limited. Patients were eligible if they had left ventricular ejection fraction (LVEF) ≥45%, NYHA functional class II–IV, BMI ≥30 kg m−2, KCCQ-CSS <90 points and objective evidence of HF based on at least one of the following criteria: (1) elevated left ventricular filling pressures (pulmonary artery wedge pressure or left ventricular end-diastolic pressure ≥15 mmHg at rest or ≥25 mmHg with exercise documented during catheterization or pulmonary artery diastolic pressure measured by implantable monitor ≥15 mmHg, assessed invasively); (2) elevated natriuretic peptide levels (with thresholds stratified based on BMI: ≥220 pg ml−1 for patients with BMI <35.0 and sinus rhythm; ≥660 pg ml−1 for patients with BMI <35.0 and persistent/permanent atrial fibrillation; ≥125 pg ml−1 for patients with BMI ≥35.0 and sinus rhythm; or ≥375 pg ml−1 for patients with BMI ≥35.0 and persistent/permanent atrial fibrillation, together with echocardiographic abnormalities (at least one of the following: (i) septal é <7 cm s−1 or lateral é < 10 cm s−1 or average E/é ≥15; (ii) pulmonary artery systolic pressure >35 mmHg; (iii) left atrial enlargement defined by local laboratory; and (iv) left ventricular hypertrophy with septal thickness or posterior wall thickness ≥1.2 cm)); or (3) hospitalization for HF in the preceding 12 months plus requirement for ongoing diuretics and/or echocardiographic abnormalities (as defined above). Key exclusion criteria were previous or planned bariatric surgery, self-reported change in body weight >11 pounds (5 kg) within 90 d before randomization or SBP >160 mmHg at screening. Patients were excluded from the trial if they had a HbA1c level ≥6.5% or prior medical history of diabetes, because clinical characteristics and response to semaglutide may differ in patients with diabetes. A sister trial (STEP-HFpEF DM) is evaluating the effects of semaglutide in patients with obesity phenotype of HFpEF and diabetes (NCT04916470). The STEP-HFpEF trial was sponsored by Novo Nordisk.
BMI and weight changes
BMI was calculated as body weight in kilograms divided by height in meters squared based on measurements at baseline before randomization. Patients were stratified into BMI categories as obesity class I (BMI 30–34.9 kg m−2), class II (BMI 35–39.9 kg m−2) or class III (BMI ≥40 kg m−2). Relative changes in body weight were expressed as the difference in body weight between baseline and 52 weeks divided by baseline body weight calculated as percentage.
Outcomes
The dual primary endpoints of STEP-HFpEF were change in KCCQ-CSS and percent change in body weight from baseline to 52 weeks11,12. Confirmatory secondary endpoints included exercise function assessed by change in 6MWD, overall clinical benefit assessed using a hierarchical composite endpoint (all-cause death, HF events, several thresholds of change in KCCQ-CSS from baseline to 52 weeks and change in 6MWD ≥30 m) and change in CRP from baseline to 52 weeks. All serious adverse events and adverse events leading to premature treatment discontinuation were reported to evaluate safety and tolerability.
Statistical analysis
Baseline characteristics were evaluated according to BMI groups (30–<35, 35–<40 and ≥40 kg m−2), and tests for trend were performed across these groups. Efficacy endpoints for semaglutide compared to placebo, stratified by obesity class at baseline, were assessed using both the full analysis set (all randomized participants according to the intention-to-treat principle, regardless of treatment discontinuation) and the on-treatment data (including only patients receiving allocated study medication). Weight loss ‘dose–effect’ analyses were performed according to the magnitude of body weight change during the trial confined to the semaglutide group, because the primary objective was to examine the effects of body weight change related to semaglutide treatment rather than spontaneous or other lifestyle-related weight changes (as in the placebo group), using both intention-to-treat (primary) and on-treatment approaches. Subgroup analyses for continuous endpoints in the intention-to-treat were performed using 1,000 multiple imputations using analysis of covariance models, with treatment by BMI groups adjusted for the relevant continuous baseline variable12. Estimates from the multiple imputations were derived using Rubin’s rule. Subgroups analyses of the hierarchical composite endpoint (win ratio) were performed stratified by the obesity category, based on direct comparisons of each participant randomized to semaglutide versus each participant randomized to placebo within each BMI subgroup. For each of these participant pairs, a ‘treatment winner’ based on similar observation time was declared based on the endpoint hierarchy (as previously reported11,12). The win ratio (that is, the proportion of winners randomized to semaglutide divided by the winners randomized to placebo) was estimated independently within each BMI subgroup using 1,000 imputations. Test for equality of the BMI groups for the win ratio was performed using Cohranʼs Q-test. Subgroup analyses for continuous endpoints in relation to the secondary hypothetical estimand (on treatment with trial product) were performed using a mixed model with treatment by BMI group adjusted for the relevant continuous baseline variable, all nested within visit, and treatment by BMI groups was evaluated at week 52 using on-treatment data. The hierarchical endpoint was analyzed using prediction (single-imputed) from a mixed model using on-treatment data for each of the components and analyzed stratified as described above. All imputations for the win ratio were pertinent only to KCCQ-CSS and 6MWD, where all-cause death and HF events differed between intention-to-treat and on-treatment approaches due to the collection of events in these two trial periods. Multivariable regression analyses were performed to determine independent relationships between baseline BMI and baseline outcome measures before treatment after adjusting a priori for baseline characteristics that might confound interpretation (age, sex, NYHA class, history of atrial fibrillation and history of coronary artery disease). Multivariable linear regression was also performed to determine relationships between change in body weight and changes in study outcomes with semaglutide unadjusted and after adjusting (a priori) for age, sex, NYHA functional class, history of coronary artery disease, history of atrial fibrillation, baseline CRP and baseline NTproBNP levels. Both unadjusted and adjusted analyses included baseline body weight and relevant continuous baseline variables (for example, baseline KCCQ-CSS, 6MWD or CRP) as covariates. Change in body weight was analyzed both as a continuous variable (% change from baseline) and as an ordinal variable, including the following weight loss categories from baseline to 52 weeks: <5%, 5–<10%, 10–<15%, 15–<20% and ≥20%. A test for linearity was employed for the categorial weight change analyses. All results from statistical analyses are presented with two-sided P values and 95% CIs. Safety endpoints were analyzed using the safety analysis set (all randomized participants exposed to at least one dose of randomized treatment). Further details on the estimands, including specification of intention-to-treat and on-treatment data, statistical analyses and imputation methods to account for missing data, were previously published12. The primary estimand quantified the average change from baseline to 52 weeks in KCCQ-CSS and body weight of semaglutide 2.4 mg once weekly relative to placebo, both added to standard of care, in all randomized participants regardless of adherence to randomized treatment. P values less than 5% were considered significant, and no adjustment for multiplicity was performed.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Data will be shared with bona fide researchers submitting a research proposal approved by the independent review board. Instructions for submitting proposals can be found at https://www.novonordisk-trials.com/. Data will be made available after research completion and approval of the product and product use in the European Union and the United States. Individual participant data will be shared in datasets in a de-identified/anonymized format.
References
Redfield, M. M. & Borlaug, B. A. Heart failure with preserved ejection fraction: a review. JAMA 329, 827–838 (2023).
Borlaug, B. A., Sharma, K., Shah, S. J. & Ho, J. E. Heart failure with preserved ejection fraction: JACC Scientific Statement. J. Am. Coll. Cardiol. 81, 1810–1834 (2023).
Morgen, C. S. et al. Obesity, cardiorenal comorbidities and risk of hospitalization in patients with heart failure with preserved ejection fraction. Mayo Clin. Proc. https://doi.org/10.1016/j.mayocp.2023.07.008 (2023).
Dalos, D. et al. Functional status, pulmonary artery pressure, and clinical outcomes in heart failure with preserved ejection fraction. J. Am. Coll. Cardiol. 68, 189–199 (2016).
Kitzman, D. W. & Shah, S. J. The HFpEF obesity phenotype: the elephant in the room. J. Am. Coll. Cardiol. 68, 200–203 (2016).
Obokata, M., Reddy, Y. N. V., Pislaru, S. V., Melenovsky, V. & Borlaug, B. A. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation 136, 6–19 (2017).
Reddy, Y. N. V. et al. Characterization of the obese phenotype of heart failure with preserved ejection fraction: a RELAX trial ancillary study. Mayo Clin. Proc. 94, 1199–1209 (2019).
Reddy, Y. N. V. et al. Quality of life in heart failure with preserved ejection fraction: importance of obesity, functional capacity, and physical inactivity. Eur. J. Heart Fail. 22, 1009–1018 (2020).
Adamson, C. et al. Dapagliflozin for heart failure according to body mass index: the DELIVER trial. Eur. Heart J. 43, 4406–4417 (2022).
Borlaug, B. A. et al. Obesity and heart failure with preserved ejection fraction: new insights and pathophysiological targets. Cardiovasc. Res. 118, 3434–3450 (2023).
Kosiborod, M. N. et al. Once weekly semaglutide in heart failure with preserved ejection fraction and obesity. N. Engl. J. Med. (in the press).
Kosiborod, M. N. et al. Design and baseline characteristics of STEP-HFpEF program evaluating semaglutide in patients with obesity HFpEF phenotype. JACC Heart Fail. 11, 1000–1010 (2023).
Sorimachi, H. et al. Long-term changes in cardiac structure and function following bariatric surgery. J. Am. Coll. Cardiol. 80, 1501–1512 (2022).
Reddy, Y. N. V. et al. Hemodynamic effects of weight loss in obesity: a systematic review and meta-analysis. JACC Heart Fail. 7, 678–687 (2019).
Kitzman, D. W. et al. Effect of caloric restriction or aerobic exercise training on peak oxygen consumption and quality of life in obese older patients with heart failure with preserved ejection fraction: a randomized clinical trial. JAMA 315, 36–46 (2016).
Sorimachi, H. et al. Pathophysiologic importance of visceral adipose tissue in women with heart failure and preserved ejection fraction. Eur. Heart J. 42, 1595–1605 (2021).
Acknowledgements
This trial was sponsored by Novo Nordisk and is registered with ClinicalTrials.gov (NCT04788511). The sponsor took responsibility for activities related to trial conduct, data collection and statistical analysis. The authors are indebted to the trial participants, the investigators and trial site staff who conducted the trial. Administrative support and development of figures and tables were provided by C. McKeown and L. Ambrose of Apollo, OPEN Health Communications, and were funded by Novo Nordisk, in accordance with Good Publication Practice guidelines (https://www.ismpp.org/gpp-2022). B.A.B. is supported, in part, by National Institutes of Health (NIH) grants R01 HL128526, R01 HL162828 and U01 HL160226 and by US Department of Defense grant W81XWH2210245. M.J.D. is supported by Leicester National Institute for Health Research (NIHR) Biomedical Research Centre, Leicester General Hospital. D.W.K. is supported, in part, by NIH grants U01AG076928, R01AG078153, R01AG045551, R01AG18915, P30AG021332, U24AG059624 and U01HL160272. S.V. is supported by the Canadian Institutes of Health Research and the Heart and Stroke Foundation of Canada and holds the Canada Research Chair in Cardiovascular Surgery. M.C.P. is supported by the British Heart Foundation Centre of Research Excellence Award (RE/13/5/30177 and RE/18/6/34217+). S.J.S. was supported by research grants from the NIH (U54 HL160273, R01 HL107577, R01 HL127028, R01 HL140731 and R01 HL149423). D.W. is a member of SFB1425, funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation).
Author information
Authors and Affiliations
Contributions
The academic members (M.N.K., B.A.B., J.B., M.J.D., D.W.K., M.C.P., S.J.S. and S.V.) of the Steering Committee along with the sponsor, Novo Nordisk, conceived and designed the study. The first draft of the manuscript was prepared by B.A.B. and M.N.K. M.N.K. had full access to all study data. All authors interpreted the data, contributed to manuscript writing, approved the final version of the manuscript, vouched for data accuracy and fidelity to the protocol and had final responsibility for the decision to submit for publication.
Corresponding author
Ethics declarations
Competing interests
B.A.B. receives research support from the National Institutes of Health (NIH) and the US Department of Defense as well as research grant funding from AstraZeneca, Axon, GlaxoSmithKline, Medtronic, Mesoblast, Novo Nordisk, Rivus and Tenax Therapeutics. B.A.B. has also served as a consultant for Actelion, Amgen, Aria, Axon Therapies, BD Biosciences, Boehringer Ingelheim, Cytokinetics, Edwards Lifesciences, Eli Lilly, Imbria, Janssen, Merck, Novo Nordisk, NGM Biopharmaceuticals, NXT and VADovations and is named as an inventor (US patent no. 10,307,179) for the tools and approach for a minimally invasive pericardial modification procedure to treat heart failure. D.W.K. was supported, in part, by the Kermit Glenn Phillips II Chair in Cardiovascular Medicine and NIH grants U01AG076928, R01AG078153, R01AG045551, R01AG18915 and U01HL160272 and reports receiving honoraria as a consultant for Bayer, Corvia Medical, Boehringer Ingelheim, Ketyo, Rivus, Novo Nordisk, AstraZeneca, Pfizer and Novartis; grant funding from Novartis, Bayer, Novo Nordisk, Rivus, Pfizer and AstraZeneca; and stock ownership in Gilead Sciences. M.J.D. has acted as a consultant, advisory board member and speaker for Boehringer Ingelheim, Eli Lilly, Novo Nordisk and Sanofi; an advisory board member and speaker for AstraZeneca; an advisory board member for Pfizer, Medtronic and ShouTi Pharma Inc.; and a speaker for Novartis, Sanofi and Amgen. M.D. has received grants in support of investigator and investigator-initiated trials from AstraZeneca, Sanofi-Aventis, Eli Lilly, Boehringer Ingelheim, Janssen and Novo Nordisk. J.B. is a consultant to Abbott, American Regent, Amgen, Applied Therapeutic, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Cardiac Dimension, Cardior, CVRx, Cytokinetics, Edwards, Element Science, Innolife, Impulse Dynamics, Imbria, Inventiva, Lexicon, Eli Lilly, LivaNova, Janssen, Medtronics, Merck, Occlutech, Novartis, Novo Nordisk, Pfizer, Pharmacosmos, Pharmain, Roche, Sequana, SQ Innovation, 3live and Vifor. M.C.P. has received research grants or consultancy fees from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Napp Pharmaceuticals, Novartis, Novo Nordisk, Pharmacosmos, Roche and SQ Innovations; has served on committees for AbbVie, Akero, Alnylam, AstraZeneca, Bayer, Boehringer Ingelheim, GlaxoSmithKline, New Amsterdam, Novo Nordisk, Resverlogix and Teikoku; and is Director of Global Clinical Trial Partners. S.J.S. reports receiving consulting fees from Abbott, AstraZeneca, Amgen, Aria CV, Axon Therapies, Bayer, Boehringer Ingelheim, Boston Scientific, Bristol Myers Squibb, Cyclerion, Cytokinetics, Edwards Lifesciences, Eidos, Imara, Impulse Dynamics, Intellia, Ionis, Eli Lilly, Merck, Metabolic Flux, MyoKardia, NGM Biopharmaceuticals, Novartis, Novo Nordisk, Pfizer, Prothena, Regeneron, Rivus, Sardocor, Shifamed, Tenax, Tenaya and United Therapeutics. S.V. reports speaking honoraria and/or consulting fees from Abbott, AstraZeneca, Bayer, Boehringer Ingelheim, Novo Nordisk, Novartis, Merck, PhaseBio, HLS Therapeutics, Amarin, Eli Lilly, Janssen, Pfizer, TIMI, Canadian Medical and Surgical Knowledge Translation Research Group. W.A. reports honoraria and/or consulting fees from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Novartis and Novo Nordisk. F.Z.A. reports honoraria and/or consulting fees from Abbott, AstraZeneca, Medtronic, Novo Nordisk, Occlutech, Pharmacosmos and Vifor. V.C. reports speaker fees from AstraZeneca, Boehringer Ingelheim, Cipla, Dr. Reddy’s, Lupin, Novartis, Novo Nordisk, Mankind, Pfizer, Sanofi, Sun Pharma and Torrent. J.E. reports research support for trial leadership from American Regent, Applied Therapeutics, Bayer, Cytokinetics, Merck and Novo Nordisk; honoraria for consultancy from AstraZeneca, Bayer, Boehringer Ingelheim, Novartis, Novo Nordisk and Otsuka; and service as an advisor to US2.ai. M.F. reports no conflicts of interest. H.I. reports honoraria and/or consulting fees from AstraZeneca, Bayer, Boehringer Ingelheim, Daiichi-Sankyo, Mochida, Novartis and Novo Nordisk. M.L. reports honoraria and/or consulting fees from AstraZeneca, Bayer, Boehringer Ingelheim, Ewopharma, Gedeon Richter, Novartis, Novo Nordisk, Roche and Servier. V.M. reports consulting fees from Bayer, Merck Sharp & Dohme and Novo Nordisk; research grants from Regeneron; and research support from the National Institute for Research of Metabolic and Cardiovascular Diseases (Program EXCELES, project no. LX22NPO5104), funded by the European Union–Next Generation EU. J.N. reports honoraria and/or consulting fees from Alleviant, AstraZeneca, Bayer, Boehringer Ingelheim, Cytokinetics, Pfizer, Novartis, Novo Nordisk, Rovi and Vifor. E.P. reports honoraria from Novo Nordisk. M. Schou reports speaker fees from AstraZeneca, Boehringer Ingelheim, Novartis and Novo Nordisk. M. Senni reports honoraria and/or consulting fees from Abbott, AstraZeneca, Bayer, Boehringer Ingelheim, Merck, Merck Sharp & Dohme, Novartis, Novo Nordisk and Vifor. P.v.d.M. reports institutional payments for consultancy fees and/or grants from AstraZeneca, Boehringer Ingelheim, BridgeBio, Ionis, Novartis, Novo Nordisk, Pfizer, Pharmacosmoc, Pharma Nord and Vifor. D.V.L. reports honoraria and/or consulting fees from AstraZeneca, Bayer, Boehringer Ingelheim, Merck Sharp & Dohme, Novartis, Novo Nordisk, Recardio, Sanofi, Sanova and Vaxxinity. D.W. reports consultancy fees from Novo Nordisk. S.R., E.B., M.N.E., G.K.H. and D.V.M. are employees of and hold shares in Novo Nordisk A/S. M.N.K. served as a consultant or on an advisory board for 35Pharma, Alnylam, Amgen, Applied Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Cytokinetics, Dexcom, Eli Lilly, Esperion Therapeutics, Janssen, Lexicon Pharmaceuticals, Merck (Diabetes and Cardiovascular), Novo Nordisk, Pharmacosmos, Pfizer, scPharmaceuticals, Sanofi, Structure Therapeutics, Vifor and Youngene Therapeutics. M.N.K. has also received research grants from AstraZeneca, Boehringer Ingelheim and Pfizer; holds stock in Artera Health and Saghmos Therapeutics; has received honoraria from AstraZeneca, Boehringer Ingelheim and Novo Nordisk; and has received other research support from AstraZeneca.
Peer review
Peer review information
Nature Medicine thanks Marco Metra, Jennifer Ho, Anuradha Lala-Trindade and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary handling editor: Jennifer Sargent, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1
Participant flow diagram.
Extended Data Fig. 2
Relationship between the magnitude of body weight reduction on semaglutide with change in KCCQ-CSS (a); 6MWD (b); and ratio of CRP to baseline (c) in the on-treatment (per protocol) analysis. Data are point estimates and 95% CIs. Tests for trend are based on an F-test; P values are two-sided.
Supplementary information
Supplementary Information
Supplementary Table 1
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Borlaug, B.A., Kitzman, D.W., Davies, M.J. et al. Semaglutide in HFpEF across obesity class and by body weight reduction: a prespecified analysis of the STEP-HFpEF trial. Nat Med 29, 2358–2365 (2023). https://doi.org/10.1038/s41591-023-02526-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-023-02526-x
This article is cited by
-
Pharmacotherapy for obesity: moving towards efficacy improvement
Diabetology & Metabolic Syndrome (2024)
-
Risk Stratification and Treatment of Obesity for Primary and Secondary Prevention of Cardiovascular Disease
Current Atherosclerosis Reports (2024)
-
Putting More Weight on Obesity Trials in Heart Failure
Current Heart Failure Reports (2024)
-
Poly-Agonist Pharmacotherapies for Metabolic Diseases: Hopes and New Challenges
Drugs (2024)
-
Worth Their Weight? An Update on New and Emerging Pharmacologic Agents for Obesity and Their Potential Role for Persons with Cardiac Conditions
Current Cardiology Reports (2024)