Abstract
The most common form of genetic heart disease is hypertrophic cardiomyopathy (HCM), which is caused by variants in cardiac sarcomeric genes and leads to abnormal heart muscle thickening. Complications of HCM include heart failure, arrhythmia and sudden cardiac death. The dominant-negative c.1208G>A (p.R403Q) pathogenic variant (PV) in β-myosin (MYH7) is a common and well-studied PV that leads to increased cardiac contractility and HCM onset. In this study we identify an adenine base editor and single-guide RNA system that can efficiently correct this human PV with minimal bystander editing and off-target editing at selected sites. We show that delivery of base editing components rescues pathological manifestations of HCM in induced pluripotent stem cell cardiomyocytes derived from patients with HCM and in a humanized mouse model of HCM. Our findings demonstrate the potential of base editing to treat inherited cardiac diseases and prompt the further development of adenine base editor-based therapies to correct monogenic variants causing cardiac disease.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data needed to evaluate the conclusions in the paper are present in the paper, extended data and supplementary material. Raw and analyzed RNA-seq data generated during this study are available in the Gene Expression Omnibus repository (http://www.ncbi.nlm.nih.gov/geo/) and are accessible through Gene Expression Omnibus series accession number GSE201755. DNA sequencing files can be accessed at the National Center for Biotechnology Information Sequence Read Archive (NCBI SRA) with accession code PRJNA902011. The mm10 reference genome is available at https://www.ncbi.nlm.nih.gov/assembly/GCF_000001635.20/.
Code availability
The MATLAB code used to perform contractile force measurements of iPSC-CMs has been deposited to GitHub: https://github.com/DarisaLLC/Cardio.
References
Maron, B. J. Clinical course and management of hypertrophic cardiomyopathy. N. Engl. J. Med 379, 655–668 (2018).
Semsarian, C., Ingles, J., Maron, M. S. & Maron, B. J. New perspectives on the prevalence of hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 65, 1249–1254 (2015).
Trivedi, D. V., Adhikari, A. S., Sarkar, S. S., Ruppel, K. M. & Spudich, J. A. Hypertrophic cardiomyopathy and the myosin mesa: viewing an old disease in a new light. Biophys. Rev. 10, 27–48 (2018).
Geisterfer-Lowrance, A. A. et al. A molecular basis for familial hypertrophic cardiomyopathy: a beta cardiac myosin heavy chain gene missense mutation. Cell 62, 999–1006 (1990).
Tyska, M. J. et al. Single-molecule mechanics of R403Q cardiac myosin isolated from the mouse model of familial hypertrophic cardiomyopathy. Circ. Res. 86, 737–744 (2000).
Sarkar, S. S. et al. The hypertrophic cardiomyopathy mutations R403Q and R663H increase the number of myosin heads available to interact with actin. Sci. Adv. 6, eaax0069 (2020).
Gaudelli, N. M. et al. Programmable base editing of A*T to G*C in genomic DNA without DNA cleavage. Nature 551, 464–471 (2017).
Komor, A. C., Kim, Y. B., Packer, M. S., Zuris, J. A. & Liu, D. R. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 533, 420–424 (2016).
Koblan, L. W. et al. In vivo base editing rescues Hutchinson-Gilford progeria syndrome in mice. Nature 589, 608–614 (2021).
Suh, S. et al. Restoration of visual function in adult mice with an inherited retinal disease via adenine base editing. Nat. Biomed. Eng. 5, 169–178 (2021).
Chemello, F. et al. Precise correction of Duchenne muscular dystrophy exon deletion mutations by base and prime editing. Sci. Adv. 7, eabg4910 (2021).
Reichart, D. et al. Efficient in vivo genome editing prevents hypertrophic cardiomyopathy in mice. Nat. Med. (2022).
Koblan, L. W. et al. Improving cytidine and adenine base editors by expression optimization and ancestral reconstruction. Nat. Biotechnol. 36, 843–846 (2018).
Richter, M. F. et al. Phage-assisted evolution of an adenine base editor with improved Cas domain compatibility and activity. Nat. Biotechnol. 38, 883–891 (2020).
Walton, R. T., Christie, K. A., Whittaker, M. N. & Kleinstiver, B. P. Unconstrained genome targeting with near-PAMless engineered CRISPR-Cas9 variants. Science 368, 290–296 (2020).
Nishimasu, H. et al. Engineered CRISPR-Cas9 nuclease with expanded targeting space. Science 361, 1259–1262 (2018).
Kleinstiver, B. P. et al. High-fidelity CRISPR-Cas9 nucleases with no detectable genome-wide off-target effects. Nature 529, 490–495 (2016).
Marian, A. J. & Braunwald, E. Hypertrophic cardiomyopathy: genetics, pathogenesis, clinical manifestations, diagnosis, and therapy. Circ. Res 121, 749–770 (2017).
Concordet, J. P. & Haeussler, M. CRISPOR: intuitive guide selection for CRISPR/Cas9 genome editing experiments and screens. Nucleic. Acid. Res. 46, W242–W245 (2018).
Pua, C. J. et al. Genetic studies of hypertrophic cardiomyopathy in Singaporeans identify variants in TNNI3 and TNNT2 that are common in Chinese patients. Circ. Genom. Precis. Med. 13, 424–434 (2020).
Toepfer, C. N. et al. Myosin sequestration regulates sarcomere function, cardiomyocyte energetics, and metabolism, informing the pathogenesis of hypertrophic cardiomyopathy. Circulation 141, 828–842 (2020).
Cohn, R. et al. A contraction stress model of hypertrophic cardiomyopathy due to sarcomere mutations. Stem Cell Rep. 12, 71–83 (2019).
Vakrou, S. & Abraham, M. R. Hypertrophic cardiomyopathy: a heart in need of an energy bar? Front. Physiol. 5, 309 (2014).
Lyons, G. E., Schiaffino, S., Sassoon, D., Barton, P. & Buckingham, M. Developmental regulation of myosin gene expression in mouse cardiac muscle. J. Cell Biol. 111, 2427–2436 (1990).
Geisterfer-Lowrance, A. A. et al. A mouse model of familial hypertrophic cardiomyopathy. Science 272, 731–734 (1996).
Ma, S. et al. Efficient correction of a hypertrophic cardiomyopathy mutation by ABEmax-NG. Circ. Res. 129, 895–908 (2021).
Ishikawa, K., Weber, T. & Hajjar, R. J. Human cardiac gene therapy. Circ. Res. 123, 601–613 (2018).
Zettler, J., Schutz, V. & Mootz, H. D. The naturally split Npu DnaE intein exhibits an extraordinarily high rate in the protein trans-splicing reaction. FEBS Lett. 583, 909–914 (2009).
Prasad, K. M., Xu, Y., Yang, Z., Acton, S. T. & French, B. A. Robust cardiomyocyte-specific gene expression following systemic injection of AAV: in vivo gene delivery follows a Poisson distribution. Gene Ther. 18, 43–52 (2011).
Mendell, J.R. et al. Current Clinical Applications of In Vivo Gene Therapy with AAVs. Mol. Ther. 29, 464–488 (2021).
Teekakirikul, P. et al. Cardiac fibrosis in mice with hypertrophic cardiomyopathy is mediated by non-myocyte proliferation and requires Tgf-beta. J. Clin. Invest. 120, 3520–3529 (2010).
Pinto, A. R. et al. Revisiting cardiac cellular composition. Circ. Res. 118, 400–409 (2016).
Jiang, J., Wakimoto, H., Seidman, J. G. & Seidman, C. E. Allele-specific silencing of mutant Myh6 transcripts in mice suppresses hypertrophic cardiomyopathy. Science 342, 111–114 (2013).
Pare, J. A., Fraser, R. G., Pirozynski, W. J., Shanks, J. A. & Stubington, D. Hereditary cardiovascular dysplasia. A form of familial cardiomyopathy. Am. J. Med. 31, 37–62 (1961).
Fananapazir, L. & Epstein, N. D. Genotype-phenotype correlations in hypertrophic cardiomyopathy. Insights provided by comparisons of kindreds with distinct and identical beta-myosin heavy chain gene mutations. Circulation 89, 22–32 (1994).
Ho, C. Y. et al. Genotype and lifetime burden of disease in hypertrophic cardiomyopathy: Insights from the Sarcomeric Human Cardiomyopathy Registry (SHaRe). Circulation 138, 1387–1398 (2018).
Carroll, K. J. et al. A mouse model for adult cardiac-specific gene deletion with CRISPR/Cas9. Proc. Natl Acad. Sci. USA 113, 338–343 (2016).
Maron, B. J., Yeates, L. & Semsarian, C. Clinical challenges of genotype positive (+)-phenotype negative (-) family members in hypertrophic cardiomyopathy. Am. J. Cardiol. 107, 604–608 (2011).
Green, E. M. et al. A small-molecule inhibitor of sarcomere contractility suppresses hypertrophic cardiomyopathy in mice. Science 351, 617–621 (2016).
Stern, J. A. et al. A small molecule inhibitor of sarcomere contractility acutely relieves left ventricular outflow tract obstruction in feline hypertrophic cardiomyopathy. PLoS ONE 11, e0168407 (2016).
Ladage, D., Ishikawa, K., Tilemann, L., Muller-Ehmsen, J. & Kawase, Y. Percutaneous methods of vector delivery in preclinical models. Gene Ther. 19, 637–641 (2012).
Cheng, Q. et al. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR-Cas gene editing. Nat. Nanotechnol. 15, 313–320 (2020).
Banskota, S. et al. Engineered virus-like particles for efficient in vivo delivery of therapeutic proteins. Cell 185, 250–265 e216 (2022).
Tabebordbar, M. et al. Directed evolution of a family of AAV capsid variants enabling potent muscle-directed gene delivery across species. Cell 184, 4919–4938 e4922 (2021).
Weinmann, J. et al. Identification of a myotropic AAV by massively parallel in vivo evaluation of barcoded capsid variants. Nat. Commun. 11, 5432 (2020).
Lompre, A. M. et al. Species- and age-dependent changes in the relative amounts of cardiac myosin isoenzymes in mammals. Dev. Biol. 84, 286–290 (1981).
Desai, M. Y. et al. Study design and rationale of VALOR-HCM: evaluation of mavacamten in adults with symptomatic obstructive hypertrophic cardiomyopathy who are eligible for septal reduction therapy. Am. Heart J. 239, 80–89 (2021).
Saberi, S. et al. Mavacamten favorably impacts cardiac structure in obstructive hypertrophic cardiomyopathy: EXPLORER-HCM Cardiac Magnetic Resonance Substudy Analysis. Circulation 143, 606–608 (2021).
Keam, S.J. Mavacamten: First Approval. Drugs 82, 1127–1135 (2022).
Ho, C. Y. et al. Evaluation of mavacamten in symptomatic patients with nonobstructive hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 75, 2649–2660 (2020).
Murphy, E. & Steenbergen, C. Gender-based differences in mechanisms of protection in myocardial ischemia-reperfusion injury. Cardiovasc. Res. 75, 478–486 (2007).
Ran, F. A. et al. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 8, 2281–2308 (2013).
Huang, T. P. et al. Circularly permuted and PAM-modified Cas9 variants broaden the targeting scope of base editors. Nat. Biotechnol. 37, 626–631 (2019).
Levy, J. M. et al. Cytosine and adenine base editing of the brain, liver, retina, heart and skeletal muscle of mice via adeno-associated viruses. Nat. Biomed. Eng. 4, 97–110 (2020).
Burridge, P. W. et al. Chemically defined generation of human cardiomyocytes. Nat. Method. 11, 855–860 (2014).
Correia, C. et al. Distinct carbon sources affect structural and functional maturation of cardiomyocytes derived from human pluripotent stem cells. Sci. Rep. 7, 8590 (2017).
Kluesner, M. G. et al. EditR: a method to quantify base editing from Sanger sequencing. CRISPR J. 1, 239–250 (2018).
Atmanli, A. et al. Cardiac myoediting attenuates cardiac abnormalities in human and mouse models of Duchenne muscular dystrophy. Circ. Res. 129, 602–616 (2021).
Kijlstra, J. D. et al. Integrated analysis of contractile kinetics, force generation, and electrical activity in single human stem cell-derived cardiomyocytes. Stem Cell Rep. 5, 1226–1238 (2015).
Doench, J. G. et al. Optimized sgRNA design to maximize activity and minimize off-target effects of CRISPR-Cas9. Nat. Biotechnol. 34, 184–191 (2016).
Clement, K. et al. CRISPResso2 provides accurate and rapid genome editing sequence analysis. Nat. Biotechnol. 37, 224–226 (2019).
Miura, H., Quadros, R. M., Gurumurthy, C. B. & Ohtsuka, M. Easi-CRISPR for creating knock-in and conditional knockout mouse models using long ssDNA donors. Nat. Protoc. 13, 195–215 (2018).
Creed, H. A. & Tong, C. W. Preparation and identification of cardiac myofibrils from whole heart samples. Methods Mol. Biol. 2319, 15–24 (2021).
Cui, M. & Olson, E. N. Protocol for single-nucleus transcriptomics of diploid and tetraploid cardiomyocytes in murine hearts. STAR Protoc. 1, 100049 (2020).
Zhou, Y. et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 10, 1523 (2019).
Acknowledgements
We thank the members of the E.N.O. laboratory for helpful discussions; J. C. Wu and the Stanford Cardiovascular Institute for providing the HCM patient-derived iPSCs; C. Seidman and H. Wakimoto for suggestions on intrathoracic AAV9 delivery; T. Lanigan, H. Kopera and R. Agate from the University of Michigan Vector Core for rAAV9 production; C. Llamas and P. Mishra from the Children’s Medical Center Research Institute for help with Seahorse assays; J. Cabrera and S. Vargas for graphics; D. Martin from Envigo for custom chow consultation; J. Xu and Y. J. Kim from the Children’s Medical Center Research Institute for performing the Illumina NextSeq sequencing; C. Rodriguez-Caycedo for assistance with iPSCs; the UT Southwestern McDermott Center Sanger Sequencing Core; the UT Southwestern McDermott Center Next-Generation Sequencing Core; the UT Southwestern Flow Cytometry Core; and J. Shelton from the Molecular Histopathology Core for help with histology. This work was supported by grants from the National Institutes of Health (R01HL130253, P50HD087351 and R01HL157281 to E.N.O. and R.B.-D.; F30HL163915 to A.C.C.), the American Heart Association (907611 to A.C.C.), the Foundation Leducq Transatlantic Networks of Excellence in Cardiovascular Research and the Robert A. Welch Foundation (grant 1-0025 to E.N.O.). The E.N.O. laboratory is supported by CureHeart, the British Heart Foundation’s Big Beat Challenge Award (BBC/F/21/220106).
Author information
Authors and Affiliations
Contributions
A.C.C., R.B.-D. and E.N.O. conceptualized the project and designed the experiments. A.C.C., F.C., H.L. and A.A. conducted in vitro iPSC-CM experiments. A.C.C., M.C., F.C., H.L. and Y.Z. conducted in vivo experiments. W.T. performed mouse echocardiography. J.R.M. performed mouse zygote injections. K.C., A.C.C. and L.X. performed bioinformatics analysis. A.C.C., N.L., R.B.-D. and E.N.O. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
E.N.O. is a consultant for Vertex Pharmaceuticals and Tenaya Therapeutics. The other authors declare no competing interests.
Peer review
Peer review information
Nature Medicine thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Anna Maria Ranzoni, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
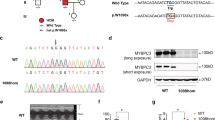
Extended Data Fig. 1 Generation of isogenic HD403/+ and HD403/403 iPSCs by homology-directed repair.
a, Using iPSCs derived from a healthy donor (HDWT), the MYH7 p.R403Q (c.1208 G > A) variant was introduced by CRISPR-Cas9-based homology-directed repair (HDR) using SpCas9, a sgRNA (spacer sequence colored in green, PAM sequence colored in gold), and a single-stranded oligodeoxynucleotide (ssODN) donor template containing the PV. A heterozygous genotype (HD403/+) and homozygous genotype (HD403/403) were isolated. Chromatograms highlighting insertion of the PV and corresponding amino acid changes are shown for indicated genotypes. Red arrows indicate coding nucleotide 1208 and amino acid 403. b, Sanger sequencing chromatogram showing no insertion of the PV on the highly homologous MYH6 gene. Red arrow indicates coding nucleotide 1211 and amino acid 404. c, HDWT and HD403/+ iPSCs readily differentiate into CMs. Cardiac troponin I (cTnI, green) highlights CMs; nuclei (blue) are marked by DAPI (4′,6-diamidino-2-phenylindole). Scale bar, 25 μm. Similar ability for iPSCs to differentiate into CMs was found in at least three separate differentiations for each genotype. d, Ratio of MYH7 to MYH6 gene expression in HDWT and HD403/+ iPSC-CMs as measured by quantitative PCR. Data are mean ± s.d. across four separate differentiations.
Extended Data Fig. 2 Computationally determined off-target sites for h403_sgRNA with ABEmax-VRQR.
a, Genomic loci of eight candidate off-target (OT) sites (left) and alignments of eight candidate off-target sequences to the on-target protospacer (right). Nucleotides that match the protospacer are indicated with a vertical dash. Nucleotides that differ are shown for each site. Numbering of nucleotides in protospacer starts with the nucleotide immediately 5′ of the PAM as nucleotide 1. b, HTS to measure editing for all 58 adenines within the protospacers of the top 8 CRISPOR-identified candidate off-target loci. HTS was performed for ABE-treated MYH7403/+ HCM1 and MYH7403/+ HCM2 iPSCs.
Extended Data Fig. 3 Comparison of predominantly expressed mouse and human myosin heavy chain sequences.
Homology comparison for mouse α-myosin heavy chain (Myh6) and human β-myosin heavy chain (MYH7) at the amino acid level (top) and DNA sequence level (bottom) around glutamine 403. The h403_sgRNA is illustrated in green and the PAM sequence is illustrated in yellow. The pathogenic c.1208 G > A variant is located at position 16 within the canonical base editing window of positions 14–17, counting the adenine nucleotide immediately 5′ of the PAM as position 1.
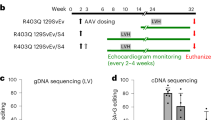
Extended Data Fig. 4 Validation of a dual AAV9 ABE system in mice.
a, Injection details for treating Myh6h403/h403 mice with ABE-AAV9 or saline. b, Kaplan-Meier curve for Myh6WT mice (n = 7; 4 male, 3 female), Myh6h403/+ mice (n = 8; 2 male, 6 female), Myh6h403/h403 mice (n = 6; 1 male, 5 female), and ABE-treated Myh6h403/h403 mice at a low (AAV LOW, n = 3; 1 male, 2 female) or high dose (AAV HIGH, n = 5; 4 male, 1 female). Median lifespans: Myh6WT and Myh6h403/+ mice, >40 days; Myh6h403/h403 mice, 7 days; AAV LOW Myh6h403/h403 mice, 9 days (1.3-fold longer, P = 0.0201); AAV HIGH Myh6h403/h403 mice, 15 days (2.1-fold longer, P = 0.0014). *P < 0.05, **P < 0.01 by log-rank (Mantel–Cox) test for AAV LOW Myh6h403/h403 mice and AAV HIGH Myh6h403/h403 mice, each, compared to Myh6h403/h403 mice. c, Sanger sequencing chromatograms for a Myh6h403/h403 mouse and a AAV HIGH Myh6h403/h403 mouse showing 35% on-target editing of the target pathogenic adenine at the cDNA level. d, Four-chamber sectioning and Masson’s trichrome staining of a AAV HIGH Myh6h403/h403 male mouse at 15 days of age. No other replications were possible as all other pups (5 total) were cannibalized before hearts could be collected.
Extended Data Fig. 5 Serial echocardiograms following dual AAV9 ABE editing of Myh6h403/+ mice.
a–f, Left ventricular anterior wall thickness at diastole (a) left ventricular posterior wall thickness at diastole (b), left ventricular internal diameter at diastole (c) and systole (d), ejection fraction (e), and fractional shortening (f), of Myh6WT mice, Myh6h403/+ mice, or ABE-treated Myh6h403/+ mice from 8–16 weeks of age. n = 5 male mice for each group. Exact P values can be found in Table 1. Data are mean ± s.e.m. *P < 0.05, **P < 0.01 by Student’s unpaired two-sided t-test for Myh6WT mice compared to Myh6h403/+ mice (black) and ABE-treated Myh6h403/+ mice compared to Myh6h403/+ mice (green). g, Representative M-mode images for Myh6WT mice, Myh6h403/+ mice, and ABE-treated Myh6h403/+ mice at 16 weeks of age.
Extended Data Fig. 6 Genomic and proteomic analysis of select tissues following dual AAV9 ABE editing.
a, Viral copy numbers for the N terminal AAV and C terminal AAV were quantified from the right atrium (RA), right ventricle (RV), left atrium (LA), left ventricle (LV), lung, liver, spleen, and quadriceps muscle (Quad) from ABE-treated Myh6h403/+ mice at 16 weeks of age. b, The percentage of A to G editing was determined by HTS of genomic DNA in the RA, RV, LA, LV, lung, liver, spleen and Quad from ABE-treated and saline-injected Myh6h403/+ mice. c, The percentage decrease in mutant transcripts in the RA, RV, LA, and LV was determined by HTS of cDNA from ABE-treated and saline-injected Myh6h403/+ mice. The percentage decrease was greater in the RV (22.7%, P = 0.0202) and the LV (26.7%, P = 0.00157) compared to the LA (12.9%). d, Cardiac myofibrils were isolated from Myh6WT mice, Myh6h403/+ mice, and ABE-treated Myh6h403/+ mice, run on a 4–20% polyacrylamide gel, and stained with Coomassie G-250. Key sarcomeric proteins are marked, including titin, myosin heavy chain (MHC), myosin binding protein C (MyBP-C), actin, cardiac troponin T (cTnT), cardiac tropomyosin (cTm), and cardiac troponin I (cTnI). Sizes for ladder markings are in kDa. Relative protein amounts for each key sarcomeric protein are normalized to WT. Data are mean ± s.d. *P < 0.05 by Student’s unpaired two-sided t-test, n = 3 male mice for each group.
Extended Data Fig. 7 RNA-sequencing analysis of dual AAV9 ABE editing of Myh6h403/+ mice.
Volcano plot showing fold-change and p-value of genes up-regulated (red) and down-regulated (blue) in Myh6h403/+ mice compared to Myh6WT mice (top), ABE-treated Myh6h403/+ mice compared to Myh6h403/+ mice (middle), and ABE-treated Myh6h403/+ mice compared to Myh6WT mice (bottom). P-adjust < 0.01 and fold-change > 2.0 were used for cutoffs; p-adjust values are calculated by two-tailed Wilcoxon rank-sum test. n = 3 male mice for each group.
Supplementary information
Supplementary Information
Supplementary Note 1, Figs. 1 and 2 and Table 1.
Source data
Source Data Fig. 1
Numerical source data.
Source Data Fig. 2
Numerical source data.
Source Data Fig. 4
Numerical source data.
Source Data Fig. 5
Numerical source data.
Source Data Extended Data Fig. 1
Numerical source data.
Source Data Extended Data Fig. 2
Numerical source data.
Source Data Extended Data Fig. 4
Numerical source data.
Source Data Extended Data Fig. 5
Numerical source data.
Source Data Extended Data Fig. 6
Numerical source data, Uncut gel.
Source Data Extended Data Fig. 6
Numerical source data, Uncut gel.
Source Data Extended Data Fig. 7
Numerical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chai, A.C., Cui, M., Chemello, F. et al. Base editing correction of hypertrophic cardiomyopathy in human cardiomyocytes and humanized mice. Nat Med 29, 401–411 (2023). https://doi.org/10.1038/s41591-022-02176-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-022-02176-5
This article is cited by
-
Strategies for non-viral vectors targeting organs beyond the liver
Nature Nanotechnology (2024)
-
Precise genome-editing in human diseases: mechanisms, strategies and applications
Signal Transduction and Targeted Therapy (2024)
-
Base editing effectively prevents early-onset severe cardiomyopathy in Mybpc3 mutant mice
Cell Research (2024)
-
CRISPR gene-editing therapies for hypertrophic cardiomyopathy
Nature Medicine (2023)
-
Drug delivery systems for CRISPR-based genome editors
Nature Reviews Drug Discovery (2023)