Abstract
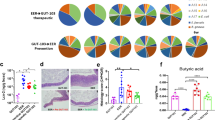
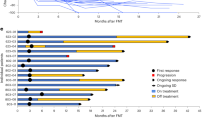
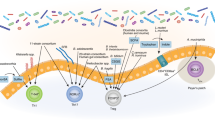
We report the first case series of immune checkpoint inhibitors (ICI)-associated colitis successfully treated with fecal microbiota transplantation, with reconstitution of the gut microbiome and a relative increase in the proportion of regulatory T-cells within the colonic mucosa. These preliminary data provide evidence that modulation of the gut microbiome may abrogate ICI-associated colitis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
Patient-related data not included in the paper were generated as part of clinical trials and may be subject to patient confidentiality. Any data and materials that can be shared will be released via a Material Transfer Agreement. Sequence data has been deposited at the European Genome-phenome Archive (EGA), which is hosted by the EBI and the CRG, under accession number EGAS00001003217.
Change history
27 November 2018
In the version of this article originally published, an author was missing from the author list. Alexander J. Lazar should have been included between Jorge M. Blando and James P. Allison. The author has been added to the list, and the author contributions section has been updated to include Alexander J. Lazar’s contribution to the study. The error has been corrected in the print, PDF and HTML versions of the manuscript.
References
Michot, J. M. et al. Eur. J. Cancer 54, 139–148 (2016).
Cramer, P. & Bresalier, R. S. Curr. Gastroenterol. Rep. 19, 3 (2017).
Chen, J. H., Pezhouh, M. K., Lauwers, G. Y. & Masia, R. Am. J. Surg. Pathol. 41, 643–654 (2017).
Dadu, R., Zobniw, C. & Diab, A. Cancer J. 22, 121–129 (2016).
Bertrand, A., Kostine, M., Barnetche, T., Truchetet, M. E. & Schaeverbeke, T. BMC Med. 13, 211 (2015).
Horvat, T. Z. et al. J. Clin. Oncol. 33, 3193–3198 (2015).
Beck, K. E. et al. J. Clin. Oncol. 24, 2283–2289 (2006).
Johnston, R. L., Lutzky, J., Chodhry, A. & Barkin, J. S. Dig. Dis. Sci. 54, 2538–2540 (2009).
Minor, D. R., Chin, K. & Kashani-Sabet, M. Cancer Biother. Radiopharm. 24, 321–325 (2009).
Borody, T. J. & Khoruts, A. Nat. Rev. Gastroenterol. Hepatol. 9, 88–96 (2011).
Gopalakrishnan, V. et al. Science 359, 97–103 (2018).
Matson, V. et al. Science 359, 104–108 (2018).
Routy, B. et al. Science 359, 91–97 (2018).
Dubin, K. et al. Nat. Commun. 7, 10391 (2016).
Vetizou, M. et al. Science 350, 1079–1084 (2015).
Chaput, N. et al. Ann. Oncol. 28, 1368–1379 (2017).
Wang, F., Yin, Q., Chen, L. & Davis, M. M. Proc. Natl Acad. Sci. USA 115, 157–161 (2018).
Kappeler, A. & Mueller, C. Histol. Histopathol. 15, 167–172 (2000).
Nancey, S. et al. Gastroenterology 131, 485–496 (2006).
Png, C. W. et al. Am. J. Gastroenterol. 105, 2420–2428 (2010).
Litvak, Y., Byndloss, M. X., Tsolis, R. M. & Baumler, A. J. Curr. Opin. Microbiol. 39, 1–6 (2017).
Jenq, R. R. et al. Biol. Blood Marrow Transplant. 21, 1373–1383 (2015).
van Nood, E. et al. N. Engl. J. Med. 368, 407–415 (2013).
Caporaso, J. G. et al. ISME J. 6, 1621–1624 (2012).
Rognes, T., Flouri, T., Nichols, B., Quince, C. & Mahe, F. PeerJ 4, e2584 (2016).
Caporaso, J. G. et al. Nat. Methods 7, 335–336 (2010).
Schloss, P. D. et al. Appl. Environ. Microbiol. 75, 7537–7541 (2009).
Lozupone, C., Lladser, M. E., Knights, D., Stombaugh, J. & Knight, R. ISME J. 5, 169–172 (2011).
Stack, E. C., Wang, C., Roman, K. A. & Hoyt, C. C. Methods 70, 46–58 (2014).
Abu-Sbeih, H. et al. J. Immunother. Cancer 6, 1–11 (2018).
Acknowledgments
We are grateful for the following funding support: Andrew Sabin Family Fellows Program (private donation to J.A.W); MD Anderson Cancer Center’s Melanoma Moon Shot Program (grant no. 710499-80-111538-19, to J.A.W.); American Association for Cancer Research Stand Up to Cancer (grant no. SU2C-AACR-IRG-19-17, to J.A.W.); National Institutes of Health (grant no. R01 CA219896-01A1, to J.A.W.); National Institutes of Health (grant no. R01 HL124112, to R.R.J.); Cancer Prevention and Research Institute of Texas (grant no. RR160089, to R.R.J.).
Author information
Authors and Affiliations
Contributions
Y.W. recruited and treated patients. Y.W., D.H.W., B.A.H., V.G., K.C., H.L.D., Z.-D.J., H.A.-S. C.A.S., C.-C.C., E.R.P., A.F.-C., G.S.R., J.R.S, M.T.C., J.G., S.K.S., D.M.M., J.M.B., A.J.L., J.P.A., P.S., M.T.T., J.A.W., and R.R.J. completed stool and tissue immunohistochemistry studies and analyzed/interpreted data. Y.W., B.A.H., V.G., M.T.T., J.A.W., and R.R.J. prepared the manuscript. All authors approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
J.A.W. and V.G. are inventors on a US patent application (PCT/US17/53,717) submitted by The University of Texas MD Anderson Cancer Center that covers methods to enhance checkpoint blockade therapy by the microbiome., J.A.W. is a paid speaker for Imedex, Dava Oncology, Omniprex, Illumina, Gilead, MedImmune and Bristol Meyers Squibb. J.A.W. is a consultant / advisory board member for Roche-Genentech, Novartis, Astra-Zeneca, Glaxo Smith Klein, Bristol Meyers Squibb, Merck, and Microbiome DX. J.A.W. also receives clinical trial support from Glaxo Smith Klein, Roche-Genentech, Bristol Meyers Squibb, and Novartis. V.G. is a consultant at Microbiome DX, and ExpertConnect. R.R.J. is on the scientific advisory board for Seres Therapeutics, Inc., has consulted for Ziopharm Oncology and Microbiome Dx, and holds patents licensed to Seres Therapeutics, Inc. M.T.T. serves on the advisory board for Novartis, Seattle Genetics, and Myriad Genetics. J.A.W., P.S., and J.P.A. are members of the Parker Institute for Cancer Immunotherapy at MD Anderson Cancer Center. P.S. is a consultant for Constellation, Kite Pharma, Neon, BioAtla, Pieris Pharmaceuticals, Oncolytics Biotech, Merck, BioMx, Forty-Seven, Polaris, Apricity, Marker Therapeutics, Codiak, Jounce Therapeutics, and is also a stockholder from Jounce Therapeutics, Neon, BioAtla, Forty-Seven, Apricity, Polaris, Marker Therapeutics, Codiak, and Kite Pharma. J.P.A. is a stockholder for Jounce Therapeutics, Neon, BioAtla, Forty-Seven, Apricity, Polaris, Marker Therapeutics, Codiak, and Kite Pharma. J.P.A. is also a consultant for Jounce Therapeutics, Neon, Amgen, Forty-Seven, Apricity, Polaris, Marker Therapeutics, Codiak, BioAlta LLC, and Tvardi Therapeutics. The other authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7, Supplementary Tables 1 and 2
Rights and permissions
About this article
Cite this article
Wang, Y., Wiesnoski, D.H., Helmink, B.A. et al. Fecal microbiota transplantation for refractory immune checkpoint inhibitor-associated colitis. Nat Med 24, 1804–1808 (2018). https://doi.org/10.1038/s41591-018-0238-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-018-0238-9
This article is cited by
-
Univariable and multivariable Mendelian randomization study identified the key role of gut microbiota in immunotherapeutic toxicity
European Journal of Medical Research (2024)
-
Live bacterial therapeutics for detection and treatment of colorectal cancer
Nature Reviews Gastroenterology & Hepatology (2024)
-
Role of gut microbiome in cancer immunotherapy: from predictive biomarker to therapeutic target
Experimental Hematology & Oncology (2023)
-
Microbiota profiles in the saliva, cancerous tissues and its companion paracancerous tissues among Chinese patients with lung cancer
BMC Microbiology (2023)
-
Role of the gut microbiota in anticancer therapy: from molecular mechanisms to clinical applications
Signal Transduction and Targeted Therapy (2023)