Abstract
In development, pioneer transcription factors access silent chromatin to reveal lineage-specific gene programs. The structured DNA-binding domains of pioneer factors have been well characterized, but whether and how intrinsically disordered regions affect chromatin and control cell fate is unclear. Here, we report that deletion of an intrinsically disordered region of the pioneer factor TCF-1 (termed L1) leads to an early developmental block in T cells. The few T cells that develop from progenitors expressing TCF-1 lacking L1 exhibit lineage infidelity distinct from the lineage diversion of TCF-1-deficient cells. Mechanistically, L1 is required for activation of T cell genes and repression of GATA2-driven genes, normally reserved to the mast cell and dendritic cell lineages. Underlying this lineage diversion, L1 mediates binding of TCF-1 to its earliest target genes, which are subject to repression as T cells develop. These data suggest that the intrinsically disordered N terminus of TCF-1 maintains T cell lineage fidelity.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
ATAC-seq, RNA-seq and CUT&RUN date have been deposited in the NCBI Gene Expression Omnibus (GEO) under accession code GSE213238. The MS proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository under dataset identifier PXD043586. Proteome raw files were searched with the SwissProt mouse database (updated January 2023; https://www.uniprot.org/help/downloads). Other publicly available datasets for microarray experiments were accessed in the GEO under accession code GSE82044. Source data are provided with this paper.
Code availability
Code is available upon reasonable request.
References
Cirillo, L. A. et al. Opening of compacted chromatin by early developmental transcription factors HNF3 (FoxA) and GATA-4. Mol. Cell 9, 279–289 (2002).
Zaret, K. S. & Carroll, J. S. Pioneer transcription factors: establishing competence for gene expression. Genes Dev. 25, 2227–2241 (2011).
Lambert, S. A. et al. The human transcription factors. Cell 172, 650–665 (2018).
Verbeek, S. et al. An HMG-box-containing T cell factor required for thymocyte differentiation. Nature 374, 70–74 (1995).
Johnson, J. L. et al. Lineage-determining transcription factor TCF-1 initiates the epigenetic identity of T cells. Immunity 48, 243–257 (2018).
Emmanuel, A. O. et al. TCF-1 and HEB cooperate to establish the epigenetic and transcription profiles of CD4+CD8+ thymocytes. Nat. Immunol. 19, 1366–1378 (2018).
Wang, W. et al. TCF-1 promotes chromatin interactions across topologically associating domains in T cell progenitors. Nat. Immunol. 23, 1052–1062 (2022).
Xu, Z. et al. Cutting edge: beta-catenin-interacting Tcf1 isoforms are essential for thymocyte survival but dispensable for thymic maturation transitions. J. Immunol. 198, 3404–3409 (2017).
Zhao, X., Shan, Q. & Xue, H. H. TCF1 in T cell immunity: a broadened frontier. Nat. Rev. Immunol. 22, 147–157 (2022).
Love, J. J. et al. Structural basis for DNA bending by the architectural transcription factor LEF-1. Nature 376, 791–795 (1995).
Xing, S. et al. Tcf1 and Lef1 transcription factors establish CD8+ T cell identity through intrinsic HDAC activity. Nat. Immunol. 17, 695–703 (2016).
Wang, C., Uversky, V. N. & Kurgan, L. Disordered nucleiome: abundance of intrinsic disorder in the DNA- and RNA-binding proteins in 1121 species from Eukaryota, Bacteria and Archaea. Proteomics 16, 1486–1498 (2016).
Shin, Y. & Brangwynne, C. P. Liquid phase condensation in cell physiology and disease. Science https://doi.org/10.1126/science.aaf4382 (2017).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Boija, A. et al. Transcription factors activate genes through the phase-separation capacity of their activation domains. Cell 175, 1842–1855 (2018).
Ben Chorin, A. et al. ConSurf-DB: an accessible repository for the evolutionary conservation patterns of the majority of PDB proteins. Protein Sci. 29, 258–267 (2020).
Peng, K., Radivojac, P., Vucetic, S., Dunker, A. K. & Obradovic, Z. Length-dependent prediction of protein intrinsic disorder. BMC Bioinformatics 7, 208 (2006).
Skinner, J. J., Lim, W. K., Bedard, S., Black, B. E. & Englander, S. W. Protein hydrogen exchange: testing current models. Protein Sci. 21, 987–995 (2012).
Bai, Y., Milne, J. S., Mayne, L. & Englander, S. W. Primary structure effects on peptide group hydrogen exchange. Proteins 17, 75–86 (1993).
Connelly, G. P., Bai, Y., Jeng, M. F. & Englander, S. W. Isotope effects in peptide group hydrogen exchange. Proteins 17, 87–92 (1993).
Hosokawa, H. et al. Stage-specific action of Runx1 and GATA3 controls silencing of PU.1 expression in mouse pro-T cells. J. Exp. Med. https://doi.org/10.1084/jem.20202648 (2021).
Hu, G. et al. Transformation of accessible chromatin and 3D nucleome underlies lineage commitment of early T cells. Immunity 48, 227–242 (2018).
Rothenberg, E. V., Hosokawa, H. & Ungerback, J. Mechanisms of action of hematopoietic transcription factor PU.1 in initiation of T cell development. Front Immunol. 10, 228 (2019).
Shin, B. et al. Runx1 and Runx3 drive progenitor to T-lineage transcriptome conversion in mouse T cell commitment via dynamic genomic site switching. Proc. Natl Acad. Sci. USA https://doi.org/10.1073/pnas.2019655118 (2021).
Ungerback, J. et al. Pioneering, chromatin remodeling, and epigenetic constraint in early T cell gene regulation by SPI1 (PU.1). Genome Res. 28, 1508–1519 (2018).
Zhou, W., Gao, F., Romero-Wolf, M., Jo, S. & Rothenberg, E. V. Single-cell deletion analyses show control of pro-T cell developmental speed and pathways by Tcf7, Spi1, Gata3, Bcl11a, Erg, and Bcl11b. Sci. Immunol. 7, eabm1920 (2022).
Zhou, W. et al. Single-cell analysis reveals regulatory gene expression dynamics leading to lineage commitment in early T cell development. Cell Syst. 9, 321–337 (2019).
Schmitt, T. M. & Zuniga-Pflucker, J. C. T cell development, doing it in a dish. Immunol. Rev. 209, 95–102 (2006).
Schmitt, T. M. & Zuniga-Pflucker, J. C. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity 17, 749–756 (2002).
de Boer, J. et al. Transgenic mice with hematopoietic and lymphoid specific expression of Cre. Eur. J. Immunol. 33, 314–325 (2003).
Yang, Q. et al. TCF-1 upregulation identifies early innate lymphoid progenitors in the bone marrow. Nat. Immunol. 16, 1044–1050 (2015).
Weber, B. N. et al. A critical role for TCF-1 in T-lineage specification and differentiation. Nature 476, 63–68 (2011).
Yoshida, H. et al. The cis-regulatory atlas of the mouse immune system. Cell 176, 897–912 (2019).
Ling, K. W. et al. GATA-2 plays two functionally distinct roles during the ontogeny of hematopoietic stem cells. J. Exp. Med. 200, 871–882 (2004).
Li, Y. et al. GATA2 regulates mast cell identity and responsiveness to antigenic stimulation by promoting chromatin remodeling at super-enhancers. Nat. Commun. 12, 494 (2021).
Onodera, K. et al. GATA2 regulates dendritic cell differentiation. Blood 128, 508–518 (2016).
Tsai, F. Y. & Orkin, S. H. Transcription factor GATA-2 is required for proliferation/survival of early hematopoietic cells and mast cell formation, but not for erythroid and myeloid terminal differentiation. Blood 89, 3636–3643 (1997).
Jin, W. et al. Critical POU domain residues confer Oct4 uniqueness in somatic cell reprogramming. Sci. Rep. 6, 20818 (2016).
Boller, S. et al. Pioneering activity of the C-terminal domain of EBF1 shapes the chromatin landscape for B cell programming. Immunity 44, 527–541 (2016).
Wang, Y. et al. A prion-like domain in transcription factor EBF1 promotes phase separation and enables B cell programming of progenitor chromatin. Immunity 53, 1151–1167 (2020).
de Bruijn, M. F. & Speck, N. A. Core-binding factors in hematopoiesis and immune function. Oncogene 23, 4238–4248 (2004).
Xing, S. et al. Tle corepressors are differentially partitioned to instruct CD8+ T cell lineage choice and identity. J. Exp. Med. 215, 2211–2226 (2018).
Hosokawa, H. et al. Transcription factor PU.1 represses and activates gene expression in early T cells by redirecting partner transcription factor binding. Immunity 48, 1119–1134 (2018).
Frederick, M. A. et al. A pioneer factor locally opens compacted chromatin to enable targeted ATP-dependent nucleosome remodeling. Nat. Struct. Mol. Biol. 30, 31–37 (2023).
Minderjahn, J. et al. Mechanisms governing the pioneering and redistribution capabilities of the non-classical pioneer PU.1. Nat. Commun. 11, 402 (2020).
Xue, B., Dunbrack, R. L., Williams, R. W., Dunker, A. K. & Uversky, V. N. PONDR-FIT: a meta-predictor of intrinsically disordered amino acids. Biochim. Biophys. Acta 1804, 996–1010 (2010).
Nguyen, D., Mayne, L., Phillips, M. C. & Walter Englander, S. Reference parameters for protein hydrogen exchange rates. J. Am. Soc. Mass. Spectrom. 29, 1936–1939 (2018).
Carleton, M. et al. Signals transduced by CD3ε, but not by surface pre-TCR complexes, are able to induce maturation of an early thymic lymphoma in vitro. J. Immunol. 163, 2576–2585 (1999).
Ogilvy, S. et al. Promoter elements of vav drive transgene expression in vivo throughout the hematopoietic compartment. Blood 94, 1855–1863 (1999).
Shimshek, D. R. et al. Codon-improved Cre recombinase (iCre) expression in the mouse. Genesis 32, 19–26 (2002).
Dou, Z. et al. Autophagy mediates degradation of nuclear lamina. Nature 527, 105–109 (2015).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Aguilan, J. T., Kulej, K. & Sidoli, S. Guide for protein fold change and P value calculation for non-experts in proteomics. Mol. Omics 16, 573–582 (2020).
Buenrostro, J. D., Giresi, P. G., Zaba, L. C., Chang, H. Y. & Greenleaf, W. J. Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat. Methods 10, 1213–1218 (2013).
Fasolino, M. et al. Genetic variation in type 1 diabetes reconfigures the 3D chromatin organization of T cells and alters gene expression. Immunity 52, 257–274 (2020).
Stirling, D. R. et al. CellProfiler 4: improvements in speed, utility and usability. BMC Bioinformatics 22, 433 (2021).
Mayne, L. Hydrogen exchange mass spectrometry. Methods Enzymol. 566, 335–356 (2016).
Mayne, L. et al. Many overlapping peptides for protein hydrogen exchange experiments by the fragment separation-mass spectrometry method. J. Am. Soc. Mass. Spectrom. 22, 1898–1905 (2011).
Kan, Z. Y., Ye, X., Skinner, J. J., Mayne, L. & Englander, S. W. ExMS2: an integrated solution for hydrogen–deuterium exchange mass spectrometry data analysis. Anal. Chem. 91, 7474–7481 (2019).
Glasoe, P. K. & Long, F. A. Use of glass electrodes to measure acidities in deuterium oxide. J. Phys. Chem. 64, 188–190 (1960).
Acknowledgements
We thank helpful discussions with members of the laboratory of G.V. in addition to M. Fasolino, K. Zaret and N. Speck. We thank K. Gupta and the Johnson Foundation Structural Biology and Biophysics Core at the Perelman School of Medicine (RRID: SCR_022414) for HX–MS resources. Flow cytometry data were generated in the Penn Cytomics and Cell Sorting Shared Resource Laboratory at the University of Pennsylvania (RRID: SCR_022376), which is partially supported by P30CA016520 (Abramson Cancer Center). We thank laboratories at the University of Pennsylvania including the Marmorstein laboratory for protein purification equipment, the Berger laboratory for 6xHis-TEV bacterial expression vector, and the Lynch laboratory for Typhoon imager access. The work in this manuscript was supported by F30AI161873 (to A.V.), R01AI091627 (to I.M.), F30AI174776 (to M.A.S.), R01-CA230800 and R01-CA248041 (to R.B.F.), the Burroughs Welcome Fund, the Chan Zuckerberg Initiative Award, W. W. Smith Charitable Trust, Sloan Foundation and the NIH grants R01AI168136, R01AI168240, UC4DK112217, U01DK112217, R01HL145754, U01DK127768 and U01DA052715 (to G.V.).
Author information
Authors and Affiliations
Contributions
Concepts and experimental design were provided by N.G. and G.V. with help from A.V., I.M. and M.A.S. Experiments were conducted by N.G., I.J., A.C., M.A.S., J.A. and S.S. Analysis of genomics data was done by N.G. with help from A.R.P. Imaging and analysis were done by A.J., N.G. and E.K.F. HX experiments and analysis were done by M.A.S. and N.G. with support from L.M. Experimental support and discussion were provided by Y.Z., R.B.F., E.J.W. and H.-H.X. The paper was written by N.G. and G.V.
Corresponding author
Ethics declarations
Competing interests
E.J.W. is a member of the Parker Institute for Cancer Immunotherapy that supports research in the laboratory of E.J.W. E.J.W. is an advisor for Danger Bio, Marengo, Janssen, NewLimit, Pluto Immunotherapeutics Related Sciences, Santa Ana Bio, Synthekine and Surface Oncology. E.J.W. is a founder of and holds stock in Surface Oncology, Danger Bio and Arsenal Biosciences. The other authors declare no competing interests.
Peer review
Peer review information
Nature Immunology thanks David Weiss and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: N. Bernard, in collaboration with the Nature Immunology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 The N terminus of TCF-1 is intrinsically disordered.
a) Size exclusion chromatography purification of affinity- and ion exchange-purified recombinant TCF-1 protein expressed in E. coli. Chromatogram (left) displays measured A280 vs. elution volume for a representative injection of pooled ion exchange fractions. Vertical dashed lines indicate approximate position of fractions pooled for analysis by HX-MS. Gel image displays SDS-PAGE analysis (stained with SYPRO Orange) of final protein input to HX-MS. Chromatogram (right) displays A280 vs. elution time for repeated analysis of indicated fractions from a prior Superose 6 Increase 10/300GL run during the initial purification. b) Plots of normalized deuterium uptake (relative to measured deuterium content after 23hrs of H-to-D exchange) at each indicated measured sample pH (pHmeas) for each TCF-1 peptide observation at the indicated exchange times. For observations with technical replicates (n = 3 independent samples for pHmeas 6.0 4sec, 10sec), center line represents mean value with error bars corresponding to standard deviation. Shaded columns indicated pHmeas and time conditions where approximately equivalent exchange is expected given the pH dependency of HX rates. c) Representative mass spectra of indicated peptide observations (generated using ExMS259). Relative to the all-1H sample (treated as HX time = 0sec), the change in m/z of each centroid distribution reflects the indicated change in mass due to deuterium incorporation. d) Time-scaled, back exchange-corrected deuterium content vs. time experimental data as in Fig. 1c was fit to a stretched-exponential function for each peptide across the indicated regions of TCF-1. Boxplots of estimated kex values are approximate estimates of observed peptide-level HX rate constants. Center line of box plots is median, limits are 1st and 3rd quartiles, and whiskers are maximum and minimum values. e) Comparison of approximate predicted (random coil) kpred vs. observed kex peptide-level HX rate constants across peptides from the L1-L7 regions of TCF-1. Values for kex are as in (d). Values for kpred were estimated from the stretched-exponential fitting approach using predicted deuterium content vs. time data calculated from the predicted residue-specific HX rate constants across each respective peptide sequence under the assumption of no protection from exchange. Pearson correlation coefficient and corresponding correlation P value are displayed.
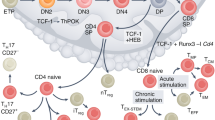
Extended Data Fig. 2 Loss of TCF-1′s L1 domain limits DN1 to DN2 transition.
a) Thy1+ CD25+ cells (b) DN1 (CD44+ CD25−), DN2 (CD44+CD25+), and DN3 (CD44- CD25+) in OP9-DLL1 co-cultures of Tcf7 cKOs transduced with EV, WT, or mutant TCF-1 (ΔL1-7) on day 13. c) Quantification of Thy1+ CD25+ cells (top) and DN2s, and DN3s by CD44 and CD25 surface expression (bottom) in OP9-DLL1 co-cultures of Tcf7 cKOs transduced with EV, WT, or mutant TCF-1 on day 13. d) DN1 (CD44− CD25−), DN2 (CD44+ CD25+), and DN3 (CD44− CD25+) cells in co-cultures of wild type (WT) ckit+ bone marrow (BM) progenitors transduced with WT TCF-1, EV, or mutant TCF-1 (ΔL1-L7) (GFP+) on OP9-DLL1 cells at day 5. e) Frequency of GFP+ (transduced) and GFP− (un-transduced) DN2s and DN3s in (D) (top). Analysis of ratio of GFP+ to GFP- Thy1+ CD25+ cells in (d) (middle). Frequency of GFP+ and GFP− Thy1+ CD25+ cells in (d) (bottom). f) DN1 (CD44− CD25−), DN2 (CD44+ CD25+) and DN3 (CD44− CD25+) cells in WT ckit+ BM progenitors transduced with WT TCF-1, ΔL1, or EV (GFP+) on OP9-DLL4 after 5 days. g) DN1 (CD44+ CD25−), DN2 (CD44+ CD25+), and DN3 (CD44− CD25+) cells in WT ckit+ BM progenitors transduced with WT TCF-1 (GFP+) on OP9-DLL1 cells (left) and OP9 cells (right) for 5 days. h) Histogram depicting TCF-1 intracellular flow cytometry in Tcf7 cKO progenitors un-transduced (Vex−) or transduced with WT TCF-1, ΔL1, or EV (Vex+) as well as WT TCF-1 sufficient progenitors. i) Frequency of B220+ Vex+ (transduced) cells in Tcf7 cKO OP9-DLL1 at day 5 (left) and 13 (right). j) Number (left) and frequency (right) of CD11b+ CD25− cells at day 7 in Tcf7 cKO OP9-DLL1 co-cultures. All cells (a-j) are pre-gated on SSC-A/FSC-A, Singlets, Live cell (Viability-), CD45+ and for a,b,c,i,j Vex+ and all data are representative of at least 3 independent experiments. In c, e, i,j, bars show mean from n = 2 independent animals, dots represent individual data points. In c, i, P values are determined by one-way ANOVA followed by Dunnett’s multiple comparison test with WT TCF-1 (P45) as a control. *P < 0.05, ** P < 0.01, *** P < 0.001, and **** P < 0.001.
Extended Data Fig. 3 GATA2 driven mast cell gene signature is unmasked in developing T cells lacking L1.
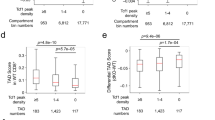
a) Heatmaps depicting gene ontology enrichment in significantly differential gene sets (adjusted P<0.05, |Log2FoldChange|>1). P values are calculated using a hypergeometric test. b) Heatmap demonstrating differentially expressed genes (adjusted P <0.05) between wild type (WT) TCF-1 transduced DN1 and DN2s from Tcf7 cKO cells on OP9-DLL1 co-cultures at day 7. P-values are calculated by the Wald test and adjusted using the Benjamini and Hochberg method. c) Principle component plot of RNA-sequencing on 293T human cell line transduced with empty vector (EV), wild type (WT) human TCF-1, and an internal deletion mutant lacking the analogous L1 region of human TCF-1; human ΔL1 (upper panel). GSEA depicts the enrichment of genes in GSE22601_IMMATURE_CD4_SINGLE_POSITIVE VS_DOUBLE_POSITIVE_THYMOCYTE_UP gene set within genes upregulated in 293T cells with human TCF-1 vs. EV. d) Heatmap depicting transcription factors differentially upregulated in ΔL1 and WT TCF-1 transduced DN2s from Tcf7 cKO cells on OP9-DLL1 co-cultures at day 7 (adjusted P<0.05 and |Log2FoldChange|>1). P-values are calculated by the Wald test and adjusted using the Benjamini and Hochberg method. e) Bar plots depicting select gene expression (in RPKM) values in DN1 and DN2s from Tcf7 cKO cells on OP9-DLL1 co-cultures at day 7. Bars represent mean RPKM values, error bars represent Standard deviation (SD), and individual data points are represented with dots.
Extended Data Fig. 4 GATA2 driven mast cell transcriptional signature is unmasked in developing T cells lacking the L1 region of TCF-1.
a–e. Representative genome browser views of counts per million normalized strand specific RNA-seq tracks at Gata2 (a.), Gata3 (b.), Thy1 (c.), Mcpt1/2/4 (d.) and Bcl11b loci (e).
Extended Data Fig. 5 The L1 domain of TCF-1 modulates binding and transcriptional outcomes in early T cell development independent of chromatin accessibility.
a) SeqLogo depicting top enriched motifs from de novo HOMER motif analysis of differentially accessible ATAC-seq peaks in WT vs. EV, ΔL1 vs. EV, and WT vs ΔL1 transduced DN1 and DN2s with non-differential peaks as background. P values are calculated using a hypergeometric test. b) Venn-diagram representing TCF-1 CUT&RUN experiments and associated unique and overlapping WT TCF-1 and ΔL1 binding events in DN1 and DN2s. c) Principal component analysis of TCF-1 CUT&RUN and chromatin accessibility measurements in DN1 and DN2s. Counts of ATAC-seq and TCF-1 binding in CUT&RUN measurements were generated across the union of all peaks across all ATAC-seq and CUT&RUN conditions. d) SeqLogo depicting top enriched motifs from de novo HOMER motif analysis of L1 dependent and independent binding events in DN1 and DN2s compared to randomly generated background. P values are calculated using a hypergeometric test. e) Heatmap depicting TCF-1 binding events measured by TCF-1 CUT&RUN and chromatin accessibility in DN1 and DN2s at differentially accessible peaks open in WT DN2 vs. DN1s.
Extended Data Fig. 6 Loss of the L1 domain of TCF-1 has limited effect on chromatin accessibility in committed T cells.
a) Principal component plot of RNA-seq on Tcf7−/− DN3 like Scid.adh cells transduced with empty vector (EV), wild type (WT) TCF-1, and internal deletion mutants: ΔL1, ΔL2, ΔL6, and ΔL7. b) Volcano plot demonstrating significantly differential genes comparing WT TCF-1 and EV (left), ΔL1 and WT TCF-1 (middle), and ΔL7 and WT TCF-1 (right) transduced Tcf7−/− DN3 cells. (adjusted P<0.05 and |Log2FoldChange|>1) P-values are calculated by the Wald test and adjusted using the Benjamini and Hochberg method. c) Heatmap depicting significantly up and down-regulated genes comparing WT TCF-1 and EV transduced Tcf7−/− DN3 cells. (adjusted P <0.05 and |Log2FC|>1). P-values are calculated by the Wald test and adjusted using the Benjamini and Hochberg method. d) Pathway enrichment analysis of differential gene sets depicted in B. P values are calculated using a hypergeometric test. e) Quantification of number of WT TCF-1 and ΔL1 binding events profiled by TCF-1 and FLAG CUT&RUN in Tcf7−/− KO DN3 cells. Bars represent mean number of binding sites from n = 2 biologically independent samples. f) Principal component plot of WT TCF-1 and ΔL1 binding events as measured in E.
Extended Data Fig. 7 Proteomics measurements suggest the interaction between RUNX1 and TCF-1 is dependent on the L1 domain.
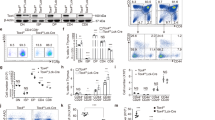
a) Representative immunofluorescence images depicting GFP tagged wild type (WT) TCF-1, ΔL1 mutant TCF-1 and empty vector (EV). DAPI staining of nuclei and overlay images are included (right). Boxplot of granularity of GFP signal in DN3 cells transduced with either EV, WT TCF-1 or ΔL1 fused with GFP. Granularity indicates the percentage of highest intensity elements of 8 pixels subtracted relative to the background (see Methods). Cells with a more granular pattern or punctate localization are indicated by a lower percentage. Center line of box plots represent median granularity, limits represent 1st and 3rd quartiles, whiskers represent maximum and minimum values, data points represent outliers. Cells analyzed per condition EV: n = 189, WT TCF-1: n = 237, ΔL1: n = 190. P values were determined by a two-tailed Mann-Whitney test: *P < 0.05, ** P < 0.01, *** P < 0.001, and **** P < 0.001. Scale bar: 4µm. b) Heatmap indicating the Z score of the log2 normalized abundance of top 100 proteins detected with a higher enrichment between DN3 cells expressing WT TCF-1 and both EV and ΔL1 in mass spectrometry of a TCF-1 immunoprecipitation in DN3 cells. c) Depiction of L1 dependent TCF-1 protein-protein interaction network identified by mass spectrometry of a TCF-1 immunoprecipitation in DN3 cells. Node size and color indicate fold change in log normalized abundance between DN3 cells expressing WT TCF-1 and EV. d) Network terms corresponding to Uniprot keywords are highlighted in the network depicted in c.
Supplementary information
Supplementary Data
Supplementary Table 1
Source data
Source Data Fig. 1
Unprocessed immunoblots.
Source Data Fig. 2
Flow cytometry gating strategy.
Source Data Fig. 5
Unprocessed immunoblots.
Source Data Fig. 6
Unprocessed immunoblots.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Goldman, N., Chandra, A., Johnson, I. et al. Intrinsically disordered domain of transcription factor TCF-1 is required for T cell developmental fidelity. Nat Immunol 24, 1698–1710 (2023). https://doi.org/10.1038/s41590-023-01599-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41590-023-01599-7
This article is cited by
-
CD4+ T cell immunity is dependent on an intrinsic stem-like program
Nature Immunology (2024)