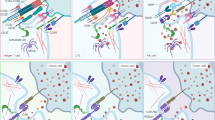
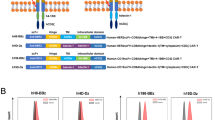
Abstract
Chimeric antigen receptor (CAR)-T cells are powerful therapeutics; however, their efficacy is often hindered by critical hurdles. Here utilizing the endocytic feature of the cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) cytoplasmic tail, we reprogram CAR function and substantially enhance CAR-T efficacy in vivo. CAR-T cells with monomeric, duplex or triplex CTLA-4 cytoplasmic tails (CCTs) fused to the C terminus of CAR exhibit a progressive increase in cytotoxicity under repeated stimulation, accompanied by reduced activation and production of proinflammatory cytokines. Further characterization reveals that CARs with increasing CCT fusion show a progressively lower surface expression, regulated by their constant endocytosis, recycling and degradation under steady state. The molecular dynamics of reengineered CAR with CCT fusion results in reduced CAR-mediated trogocytosis, loss of tumor antigen and improved CAR-T survival. CARs with either monomeric (CAR-1CCT) or duplex CCTs (CAR-2CCT) have superior antitumor efficacy in a relapsed leukemia model. Single-cell RNA sequencing and flow cytometry analysis reveal that CAR-2CCT cells retain a stronger central memory phenotype and exhibit increased persistence. These findings illuminate a unique strategy for engineering therapeutic T cells and improving CAR-T function through synthetic CCT fusion, which is orthogonal to other cell engineering techniques.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data generated or analyzed during this study are provided. Source data and statistics are provided in an Excel file in Supplementary Table 3. Processed data for genomic sequencing and gene expression are provided as processed quantifications in Supplementary Tables 1 and 2. Genomic sequencing raw data are deposited at the Gene Expression Omnibus under accession no. GSE234307. Source data are provided with this paper.
Code availability
The code used for data analysis and the generation of figures related to this study can be accessed from GitHub at https://github.com/Ariel-Xiaoyu/CAR-CCT.
References
June, C. H. & Sadelain, M. Chimeric antigen receptor therapy. N. Engl. J. Med 379, 64–73 (2018).
Orlando, E. J. et al. Genetic mechanisms of target antigen loss in CAR19 therapy of acute lymphoblastic leukemia. Nat. Med 24, 1504–1506 (2018).
Majzner, R. G. & Mackall, C. L. Tumor antigen escape from CAR T-cell therapy. Cancer Discov. 8, 1219–1226 (2018).
Schriek, P. et al. Marginal zone B cells acquire dendritic cell functions by trogocytosis. Science 375, eabf7470 (2022).
Lu, T. et al. Hijacking TYRO3 from tumor cells via trogocytosis enhances NK-cell effector functions and proliferation. Cancer Immunol. Res. 9, 1229–1241 (2021).
Huang, J. F. et al. TCR-Mediated internalization of peptide-MHC complexes acquired by T cells. Science 286, 952–954 (1999).
Hamieh, M. et al. CAR T cell trogocytosis and cooperative killing regulate tumour antigen escape. Nature 568, 112–116 (2019).
Olson, M. L. et al. Low-affinity CAR T cells exhibit reduced trogocytosis, preventing rapid antigen loss, and increasing CAR T cell expansion. Leukemia 36, 1943–1946 (2022).
Rurik, J. G. et al. CAR T cells produced in vivo to treat cardiac injury. Science 375, 91–96 (2022).
Tivol, E. A. et al. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity 3, 541–547 (1995).
Walunas, T. L. et al. CTLA-4 can function as a negative regulator of T cell activation. Immunity 1, 405–413 (1994).
Bachmann, M. F., Kohler, G., Ecabert, B., Mak, T. W. & Kopf, M. Cutting edge: lymphoproliferative disease in the absence of CTLA-4 is not T cell autonomous. J. Immunol. 163, 1128–1131 (1999).
Qureshi, O. S. et al. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science 332, 600–603 (2011).
Tekguc, M., Wing, J. B., Osaki, M., Long, J. & Sakaguchi, S. Treg-expressed CTLA-4 depletes CD80/CD86 by trogocytosis, releasing free PD-L1 on antigen-presenting cells. Proc. Natl Acad. Sci. USA https://doi.org/10.1073/pnas.2023739118 (2021).
Corse, E. & Allison, J. P. Cutting edge: CTLA-4 on effector T cells inhibits in trans. J. Immunol. 189, 1123–1127 (2012).
Walker, L. S. & Sansom, D. M. The emerging role of CTLA4 as a cell-extrinsic regulator of T cell responses. Nat. Rev. Immunol. 11, 852–863 (2011).
Benmebarek, M. R. et al. Killing mechanisms of chimeric antigen receptor (CAR) T cells. Int. J. Mol. Sci. https://doi.org/10.3390/ijms20061283 (2019).
Turtle, C. J. et al. Immunotherapy of non-Hodgkin’s lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells. Sci. Transl. Med 8, 355ra116 (2016).
Grupp, S. A. et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N. Engl. J. Med 368, 1509–1518 (2013).
Xu, Y. et al. A novel antibody-TCR (AbTCR) platform combines Fab-based antigen recognition with γδ-TCR signaling to facilitate T-cell cytotoxicity with low cytokine release. Cell Discov. 4, 62 (2018).
Wu, W. et al. Multiple signaling roles of CD3ε and its application in CAR-T cell therapy. Cell 182, 855–871 (2020).
Gust, J. et al. Endothelial activation and blood–brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells neurotoxicity associated with CD19 CAR-T cells. Cancer Discov. 7, 1404–1419 (2017).
Qureshi, O. S. et al. Constitutive clathrin-mediated endocytosis of CTLA-4 persists during T cell activation. J. Biol. Chem. 287, 9429–9440 (2012).
Sansom, D. M. Moving CTLA-4 from the trash to recycling. Science 349, 377–378 (2015).
Janman, D. et al. Regulation of CTLA‐4 recycling by LRBA and Rab11. Immunology 164, 106–119 (2021).
Gossen, M. & Bujard, H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc. Natl Acad. Sci. 89, 5547–5551 (1992).
Kao, S. H. et al. Analysis of protein stability by the cycloheximide chase assay. Bio. Protoc. https://doi.org/10.21769/BioProtoc.1374 (2015).
Martínez-Martín, N. et al. T cell receptor internalization from the immunological synapse is mediated by TC21 and RhoG GTPase-dependent phagocytosis. Immunity 35, 208–222 (2011).
Olson, M. L. et al. Low-affinity CAR T cells exhibit reduced trogocytosis, preventing rapid antigen loss, and increasing CAR T cell expansion. Leukemia 36, 1943–1946 (2022).
Bloemberg, D. et al. A high-throughput method for characterizing novel chimeric antigen receptors in Jurkat cells. Mol. Ther. Methods Clin. Dev. 16, 238–254 (2020).
Krammer, P. H. CD95’s deadly mission in the immune system. Nature 407, 789–795 (2000).
Huang, F. L., Liao, E. C., Li, C. L., Yen, C. Y. & Yu, S. J. Pathogenesis of pediatric B-cell acute lymphoblastic leukemia: molecular pathways and disease treatments. Oncol. Lett. 20, 448–454 (2020).
Gomes-Silva, D. et al. Tonic 4-1BB costimulation in chimeric antigen receptors impedes T cell survival and is vector-dependent. Cell Rep. 21, 17–26 (2017).
Long, A. H. et al. 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat. Med. 21, 581–590 (2015).
Lee, P. H. et al. Host conditioning with IL-1β improves the antitumor function of adoptively transferred T cells. J. Exp. Med. 216, 2619–2634 (2019).
Velica, P. et al. Modified hypoxia-inducible factor expression in CD8(+) T cells increases antitumor efficacy. Cancer Immunol. Res. 9, 401–414 (2021).
Maus, M. V. CD19 CAR T cells for adults with relapsed or refractory acute lymphoblastic leukaemia. Lancet 398, 466–467 (2021).
Li, Y. et al. KIR-based inhibitory CARs overcome CAR-NK cell trogocytosis-mediated fratricide and tumor escape. Nat. Med. 28, 2133–2144 (2022).
Li, G. et al. T cell antigen discovery via trogocytosis. Nat. Methods 16, 183–190 (2019).
Stojanovic, A., Fiegler, N., Brunner-Weinzierl, M. & Cerwenka, A. CTLA-4 is expressed by activated mouse NK cells and inhibits NK cell IFN-γ production in response to mature dendritic cells. J. Immunol. 192, 4184–4191 (2014).
Lougaris, V. et al. CTLA-4 regulates human natural killer cell effector functions. Clin. Immunol. 194, 43–45 (2018).
Philip, M. et al. Chromatin states define tumour-specific T cell dysfunction and reprogramming. Nature 545, 452–456 (2017).
Caruso, H. G. et al. Tuning sensitivity of CAR to EGFR density limits recognition of normal tissue while maintaining potent antitumor activity. Cancer Res. 75, 3505–3518 (2015).
Ghorashian, S. et al. Enhanced CAR T cell expansion and prolonged persistence in pediatric patients with ALL treated with a low-affinity CD19 CAR. Nat. Med. 25, 1408–1414 (2019).
Rodriguez-Marquez, P. et al. CAR density influences antitumoral efficacy of BCMA CAR T cells and correlates with clinical outcome. Sci. Adv. 8, eabo0514 (2022).
Weber, E. W. et al. Transient rest restores functionality in exhausted CAR-T cells through epigenetic remodeling. Science https://doi.org/10.1126/science.aba1786 (2021).
Guo, T., Ma, D. & Lu, T. K. Sense-and-respond payload delivery using a novel antigen-inducible promoter improves suboptimal CAR-T activation. ACS Synth. Biol. 11, 1440–1453 (2022).
Greenshpan, Y. et al. Synthetic promoters to induce immune-effectors into the tumor microenvironment. Commun. Biol. 4, 143 (2021).
Dai, X. et al. One-step generation of modular CAR-T cells with AAV-Cpf1. Nat. Methods 16, 247–254 (2019).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Yu, G. enrichplot: Visualization of Functional Enrichment Result. R package v.1.8.1. https://github.com/GuangchuangYu/enrichplot (2020).
Jespersen, H. et al. Clinical responses to adoptive T-cell transfer can be modeled in an autologous immune-humanized mouse model. Nat. Commun. 8, 707 (2017).
Satija, R., Farrell, J. A., Gennert, D., Schier, A. F. & Regev, A. Spatial reconstruction of single-cell gene expression data. Nat. Biotechnol. 33, 495–502 (2015).
Jordao, M. J. C. et al. Single-cell profiling identifies myeloid cell subsets with distinct fates during neuroinflammation. Science 363, 365–36 (2019).
Stuart, T. et al. Comprehensive integration of single-cell data. Cell 177, 1888–1902 (2019).
Becht, E. et al. Dimensionality reduction for visualizing single-cell data using UMAP. Nat. Biotechnol. https://doi.org/10.1038/nbt.4314 (2018).
Acknowledgements
We thank Drs Krause, Isufi and Bersenev for discussions on cell therapy. We thank all members of the Chen laboratory, as well as various colleagues and core facility staff at Yale for assistance and/or discussions. We thank staff at the Yale West Campus Imaging Core for their support and assistance in this work. We thank R. Glen for helpful proofreading of the manuscript. S.C. is supported by Yale SBI/Genetics Startup Fund, National Institutes of Health (NIH)/National Cancer Institute/National Institute on Drug Abuse (DP2CA238295, R01CA231112, U54CA209992-8697, R33CA225498 and RF1DA048811), U.S. Department of Defense (DOD) (W81XWH-17-1-0235, W81XWH-20-1-0072, W81XWH-21-1-0514), Cancer Research Institute (CLIP), the American Association for Cancer Research (499395, 17-20-01-CHEN), Alliance for Cancer Gene Therapy, Sontag Foundation (DSA), Pershing Square Sohn Cancer Research Alliance, Dexter Lu, Ludwig Family Foundation, Blavatnik Family Foundation and Chenevert Family Foundation. P.A.R. is supported by an NIH training grant (T32GM007499) and a Lo Fellowship. M.B.D. is supported by an NIH training grant (T32GM007205). R.D.C. is supported by an NIH MSTP training grant (T32GM007205) and National Research Service Award fellowship (F30CA250249).
Author information
Authors and Affiliations
Contributions
X.Z. designed experiments with discussion with H.C., M.B.D. and R.D.C. X.Z. and H.C. performed most of the experiments. S.Y.F., M.M., M.B. and M.B.D. assisted with the experiments. X.Z. analyzed the high-throughput data. R.D.C., P.A.R., K.T. and K.S. assisted with data analysis. A.L. and X.S. assisted with imaging analysis. X.Z. conceived the study with high-level input from S.C. S.C. secured funding and supervised the work. X.Z., H.C., R.D.C. and S.C. prepared the paper with input from all authors.
Corresponding author
Ethics declarations
Competing interests
A patent was filed by Yale University regarding the data in this study (inventors S.C. and X.Z.), which was licensed to Cellinfinity Bio, a Yale biotech startup founded by S.C. S.C. is also a (co)founder of EvolveImmune, NumericGlobal, Chen Tech and Chen Consulting, all unrelated to this study. All other authors have no competing interests.
Peer review
Peer review information
Nature Immunology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Stephanie Houston, in collaboration with the Nature Immunology team. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Characterization of CAR-T cells with CCT fusion after one round stimulation with NALM6GL cells.
a. Schematics of the workflow used to generate CAR-T cells for effector function assessment in vitro. (i) Generation of CAR-T cells. Human CD3 T cells were infected by lentiviruses carrying CAR targeting human CD22 (m971-CD28-41BB-CD3zeta). These CARs were engineered with either monomeric (CAR-1CCT), duplex (CAR-2CCT) or triplex CCTs (CAR-3CCT) fused to the C terminus of human CD22-CAR (CAR), followed by a fluorescent reporter mScarlet, separated by T2A sequences. Flag sequences were tagged at N-terminal of the CAR for surface expression detection. (ii) Round 1 co-culture. Those four groups of CAR-T cells generated in step (i) were co-culture with NALM6GL cells at variable E/T ratios for 24 hours. (iii) Round 2 co-culture. An additional round of NALM6GL cells were supplemented into the initial co-culture for 24 hours. (iv). FACS analysis. Cells from both round1 and 2 cocultures were quantified by fluorescence-activated single cell sorting (FACS). b. Representative flow cytometry results showing CD4 and CD8 staining of CAR-T cells 7 days after transduced with CAR-T constructs. c. Quantification of (b). showing the CD4 and CD8 percentage of CAR-T cells, n = 3. d. Representative flow cytometry results showing the gating strategy used in Fig. 1a–d. e. Quantification of NALM6 killing by CAR-T cells. Purified CAR-T cells were co-cultured with one round of NALM6GL cells at E/T = 1/2 for 24 hours, n = 6. f. Quantification of the increases in CAR-T cell counts after one round of NALM6GL stimulation, n = 3. g-p. Representative flow cytometry analysis and quantification of LAG3 (g,h), PD-1(i,j), TIGIT(k,l), cycling CTLA-4(m,n), recycling CTLA-4 (o) and TIM3 (p) on CAR-T cells after stimulated with NALM6GL cells at E/T = 1/2 for 24 hours. For all bar plots, data are shown as mean ± s.e.m. One-way ANOVA with Dunnett’s multiple-comparisons test is used to assess significance for figure (e), (f), (h), (j), (l) and (n). Two-way ANOVA with Tukey’s multiple-comparisons test is used to assess significance for (c) and (o). ns = p > 0.05. Exact p values are labeled. All numbers defined by ‘n’ indicate the number of biologically independent samples. Data are representative of three independent experiments performed with biological repeats.
Extended Data Fig. 2 CCT fusion enhanced CAR-T killing under repeated stimulation.
a. Representative flow cytometry analysis for Fig. 1a–d showing CAR-T killing capability under two rounds of NALM6GL stimulation. b. Quantification of input CAR-T cell counts without NALM6GL stimulation showing relatively equal amount of input CAR-T cells, n = 2, ns = p > 0.05. c. Representative flow cytometry results showing the differentiation stage, indicating by the expression of CD45RO and CD62L, of CAR-T cells after two rounds of co-culture with NALM6GL cells. d. Quantification of c. showing the CD45RO+ CD62L+ CAR-T cells in percentage, n = 6. e. Representative flow cytometry results showing the expression level of LAG3 on CAR-T cells after being stimulated with two rounds of NALM6GL. f. Quantification of (e) showing the LAG3+ CAR-T cells in percentage, n = 6. g. Representative flow cytometry analysis on the CD22 expression level of CD22high and CD22low NALM6 cells. h. Quantification of (g) showing CD22 MFI of CD22high and CD22low NALM6 cells, n = 3. i. Quantification of live NALM6 cell count after two rounds of coculturing with CAR-T cells at E/T = 1/2 by flow cytometry, n = 4. For all bar plot figures, data are shown as mean ± s.e.m. One-way ANOVA with Dunnett’s multiple-comparisons test is used to assess significance. Exact p values are labeled. All numbers defined by ‘n’ indicate the number of biologically independent samples. Data are representative of three independent experiments performed with biological repeats.
Extended Data Fig. 3 CCT fusion enables enhanced killing with low production of proinflammatory cytokines.
a. Schematics of lentiviral constructs to make CAR-T cells targeting human CD19 (FMC63-CD28-41BB-CD3zeta). CD19-CAR (19CAR) constructs were made by replacing the m971 scFv with the FMC63 scFv from Extended Data Fig. 1a. b. Representative flow cytometry analysis of 19CAR-T killing capability in vitro. c. Quantification of (b) showing CD19-CAR percentage (left panel), and counts (right panel), n = 3. d. Quantification of (b) showing NALM6GL percentage (left panel), and counts (right panel), n = 3. e. Quantification of 13 granule molecules and cytokines in CAR-T and NALM6GL cocultures. CAR-T cells were co-cultured with one round of NALM6GL cells at E/T = ½ for 24 hours. Co-culture supernatant was then collected and measured using LEGENDplex™ Human CD8/NK Panel, n = 3. f. Schematics of lentiviral constructs to make CD22 targeting CAR-T cells. In addition to the four constructs shown in Extended Data Fig. 1a, mCMV-CAR construct was generated by replacing the EFS promoter in the CAR construct with a minimal CMV promoter (mCMV). g. Representative flow cytometry analysis of CAR-T cells, pre-gated on mScarlet+ populations, showing surface expression level of CAR, indicated by Flag staining. h. Quantification of (g) showing surface flag expression in MFI, n = 3. For all bar plot figures, data are shown as mean ± s.e.m. One-way ANOVA with Dunnett’s multiple-comparisons test is used to assess significance. Exact p values are labeled. All numbers defined by ‘n’ indicate the number of biologically independent samples. Data are representative of two independent experiments performed with biological repeats.
Extended Data Fig. 4 Schematics of workflow of experiments for Fig. 2.
a. Schematics of the workflow used in Fig. 2c, d for detecting CAR endocytosis. Surface and endocytic CAR were first stained with primary anti-Flag-BV421 at 37 °C for 30 minutes, followed by a secondary anti-Rat-IgG2a-AF647 stained at 4 °C for 15 minutes. The surface, endocytic CAR and CAR- populations were differentiated based on their staining patterns of the primary and secondary antibodies. b. Schematics of the workflow used in Fig. 2e to assess the endocytosis rate of CAR. Surface CAR was initially labeled with primary anti-Flag at 4 °C, followed by incubation of CAR-T cells at 37 °C for varying durations to allow endocytosis. The remaining surface-bound CAR molecules were then stained with a secondary anti-Rat-IgG2a-AF647 antibody at 4 °C. Endocytosis was quantified by the decrease in the percentage of cells stained positive for the secondary antibody. c. Schematics of the workflow used in Fig. 2f for detecting recycling CAR. Surface and endocytic CAR were first stained with primary anti-Flag at 37 °C for 30 minutes, followed by a secondary anti-Rat-IgG2a-AF647, either stained at 4 °C for 15 minutes (baseline) or at 37 °C for 30 minutes or 60 minutes. Recycling was quantified by the increase in the percentage of cells stained positive for the secondary antibody.
Extended Data Fig. 5 CCT fusion decreased CAR-mediated trogocytosis.
a. Representative flow cytometry analysis of CAR-mediated trogocytosis. CAR-T cells were pretreated with either DMSO (control) or 1 μM Latrunculin A at 37 °C for 15 min before co-cultured with NALM6-CD22-BFP cells for 2 hours. CD22-GFP transfer onto CAR-T cells were quantified by flow cytometry. b. Quantification of (a) showing the inhibition of CD22-GFP transfer by Latrunculin A, n = 3. c. Representative flow cytometry analysis of CAR-mediated trogocytosis by CAR-J cells. CAR-J cells co-cultured with NALM6GL-CD22-BFP cells for indicated time. CD22-BFP transfer onto CAR-T cells were quantified by flow cytometry. d. Quantification of (c) showing the transfer of CD22-BFP onto CAR-J cells, n = 3. e. Schematics of the workflow used in Fig. 3h, i for detecting the degranulation of CAR-T cells. CAR-Jurkat (CAR-J) cells were co-cultured with NALM6-CD22-GFP at an E/T ratio of 1:1 for 4 hours. Both Trog+ CAR-J and Trog- CAR-J cells were sorted out based on their expression of CD22-GFP. Those cells were then co-cultured with eFluor450 labeled CAR-T cells for 2 hours for determine the expression of CD107a. f. Schematics of the CAR-T survival assay used in Fig. 4a–c. Control CAR-T cells labeled with 10 µM eFluor450 (eFluor450high), were mixed 1:1 with CAR, CAR-1CCT, CAR-2CCT or CAR-3CCT cells labeled with 1 µM eFluor450 (eFluor450low). These mixed CAR-T cells were then co-cultured with or without NALM6GL. Relative percentages of eFluor450 high and low populations gated from CAR+ (mScarlet+) cells were used for quantification of relative survival of CAR-T cells. g. Schematics of the workflow used in Fig. 4f, g. CAR-T cells were co-cultured with NALM6-CD22-GFP cells at E/T = 1/2 for 4 hours. Both Trog+ CAR-T and Trog- CAR-T cells were sorted out based on their expression of CD22-GFP, and co-cultured separately overnight before staining for Annexin V. For all bar plot figures, data are shown as mean ± s.e.m. One-way ANOVA with Dunnett’s multiple-comparisons test is used to assess significance for (d). Two-way ANOVA with Tukey’s multiple-comparisons test is used to assess significance for (b). Exact p values are labeled. All numbers defined by ‘n’ indicate the number of biologically independent samples. Data are representative of two independent experiments performed with biological repeats.
Extended Data Fig. 6 CCT fusion enhanced CAR-T survival.
a. Representative flow cytometry results showing the expression of Fas and FasL on CAR-T cells following incubations with NALM6GL cells for 0, 4 or 24 hours. b. Quantification of (a) showing the expression of Fas on CAR-T cells, n = 4. c. Quantification of (a) showing the expression of FasL on CAR-T cells, n = 4. d. Schematics of in vivo experimental workflow used in Fig. 4h–l. NSG mice (female, 6-10 weeks old) were first inoculated with 1 million NALM6GL cells intravenously (i.v.) on day 0. CAR-T cells stained with 1 µM eFluor450 were mixed with CAR-T cells stained with 10 µM eFluor450 at 1:1 ratio. Two million of these labeled cell mixtures were transferred to the leukemia NSG models on day 4. One day post CAR-T transfer, bone marrow samples were collected for flow cytometry analysis to determine the relative percentage of eFluor450low and eFluor450high populations. e. Representative flow cytometry results showing the gating strategy used in Fig. 4h–l for quantifying the relative CAR-T survival in vivo. For all bar plot figures, data are shown as mean ± s.e.m. Two-way ANOVA with Tukey’s multiple-comparisons test is used to assess significance for (b,c). Exact p values are labeled. All numbers defined by ‘n’ indicate the number of biologically independent samples. Data are representative of two independent experiments performed with biological repeats.
Extended Data Fig. 7 Characterization of CAR-CCT cells by bulk RNA sequencing.
a. Representative flow cytometry results showing the sorting purity of CAR-T cells before subjecting to RNA-seq. CAR-T cells after being stimulated with 2 rounds of NALM6GL cells were sorted as mScarlet+ GFP− cells. b. PCA analysis of all four groups of CAR-T cells at baseline without stimulation. c. PCA analysis of all four groups of CAR-T cells with 2 rounds of NALM6GL stimulation. d. Heatmap of differentially expressed genes in all four groups of CAR-T cells at baseline. All CAR-T cells were purified by flow cytometer at day 6 after lentivirus infection and expanded for 7 days before mRNA extraction for bulk RNA sequencing. Cutoff for determining differentially expressed genes is set to be p (adj) < 0.01 and |log2Fc|> 1. e-g. Quantification of LAG3(e), HHLA2(f), CD101(g) expression level on CAR-T cells at baseline using flow cytometry, n = 3. h. Volcano plot of bulk RNA sequencing showing differentially expressed genes in CAR-1CCT vs. CAR (left panel), CAR-2CCT vs. CAR (middle panel) and CAR-3CCT vs. CAR (right panel). Purified CAR-T cells were co-cultured with NALM6GL cells at E/T = 1/2. NALM6GL cells were repeatedly supplemented at 24 hrs after initial co-culture. CAR-T cells (mScarlet+) were then sorted at 72 hrs after initial co-culture for bulk RNA sequencing. Cutoff for determining differentially expressed genes is set to be p (adj) < 0.01 and |log2Fc|> 1. i. Pathway analysis by over representation analysis (ORA) showing top 10 pathways of upregulated DEGs in CAR-2CCT vs. CAR (left panel) and in CAR-3CCT vs. CAR (right panel). j. Venn diagram showing overlaps of upregulated genes as in (a). For all bar plots, data are shown as mean ± s.e.m. One-way ANOVA with Dunnett’s multiple-comparisons test is used to assess significance. Exact p values are labeled. All numbers defined by ‘n’ indicate the number of biologically independent samples. Data are representative of two independent experiments performed with biological repeats.
Extended Data Fig. 8 Characterization of CAR-CCT cells in vivo via scRNA-seq.
a. Gating strategy used for analysis shown in Fig. 5e–k. b. Heatmap showing expression profiles across 12 CAR-T cell subsets. Scaled gene expression is presented by showing 100 representative cells of each subset. c. UMAP visualization of 39,151 cells CAR-T cells via scRNA-seq profiling, split by tissue type. d. Pie chart of cell proportions between four different CAR-T groups isolated from bone marrow. e. Pie chart of cell proportions between four different CAR-T groups isolated from spleen. f. Volcano plot showing differentially expressed genes in CAR-2CCT vs. CAR-3CCT in the CD4 Teff cluster from bone marrow. Data are representative of two independent experiments performed with biological repeats.
Extended Data Fig. 9 Proposed model of titrating optimal CAR-T function with CCT fusion.
CAR fused with duplex CCTs (CAR-2CCT) demonstrated decreased surface expression, which is tightly regulated by its endocytosis, recycling, degradation in both lysosome and proteasome. Compared with the control CAR, CAR-2CCT has significant decreases in surface CAR expression, T cell activation, CAR-mediated trogocytosis, and causes less tumor antigen loss on NALM6 cells. This engineering approach effectively increases the persistence and proportion of Tcm cells in CAR-2CCT cells, leading to a remarkable improvement in their anti-tumor in vivo.
Supplementary information
Supplementary Table 1
Processed NGS data and analysis results for bulk RNA sequencing.
Supplementary Table 2
Compiled single-cell gene differential expression data.
Supplementary Video 1
Timelapse live cell imaging of trogocytosis and fratricide. Representative video showing trogocytosis and fratricide of CAR-T cells after introduction with NALM6GL-CD22-BFP cancer cells (corresponding to Fig. 3a). The white arrowhead labels a CAR-T cell that acquired CD22-BFP after an active engagement with a cancer cell, which subsequently rendered it susceptible to CAR-T fratricide afterwards, as indicated by the influx of SYTOX deep red dye. This white arrow-labeled cell then contacted another CAR-T cell, interacting at a region with enhanced CD22-BFP signal. Shortly after this active interaction, the white arrowhead-labeled cell was stained by the SYTOX deep red dye, indicating cell death. The time stamps on the top right of each image are represented as hour:minute. Scale bar, 10 µm.
Source data
Source Data
Statistical source data for all figures.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, X., Cao, H., Fang, SY. et al. CTLA-4 tail fusion enhances CAR-T antitumor immunity. Nat Immunol 24, 1499–1510 (2023). https://doi.org/10.1038/s41590-023-01571-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41590-023-01571-5