Abstract
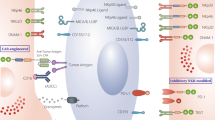
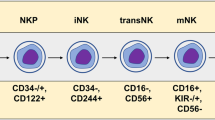
The advent of chimeric antigen receptor (CAR) T cell therapy has resulted in unprecedented long-term clearance of relapse/refractory hematological malignancies in both pediatric and adult patients. However, severe toxicities, such as cytokine release syndrome and neurotoxicity, associated with CAR T cells affect therapeutic utility; and treatment efficacies for solid tumors are still not impressive. As a result, engineering strategies that modify other immune cell types, especially natural killer (NK) cells have arisen. Owing to both CAR-dependent and CAR-independent (innate immune-mediated) antitumor killing capacity, major histocompatibility complex-independent cytotoxicity, reduced risk of alloreactivity and lack of major CAR T cell toxicities, CAR NK cells constitute one of the promising next-generation CAR immune cells that are also amenable as ‘off-the-shelf’ therapeutics. In this Review, we compare CAR T and CAR NK cell therapies, with particular focus on immunological synapses, engineering strategies and challenges.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Irvine, D. J., Maus, M. V., Mooney, D. J. & Wong, W. W. The future of engineered immune cell therapies. Science 378, 853–858 (2022).
Lee, D. W. et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol. Blood Marrow Transplant. 25, 625–638 (2019).
Lu, H., Zhao, X., Li, Z., Hu, Y. & Wang, H. From CAR-T cells to CAR-NK cells: a developing immunotherapy method for hematological malignancies. Front. Oncol. 11, 720501 (2021).
Schmidt, P., Raftery, M. J. & Pecher, G. Engineering NK Cells for CAR therapy-recent advances in gene transfer methodology. Front. Immunol. 11, 611163 (2020).
Berrien-Elliott, M. M., Jacobs, M. T. & Fehniger, T. A. Allogeneic natural killer cell therapy. Blood 141, 856–868 (2023).
Coyle, K. M., Hawke, L. G. & Ormiston, M. L. Addressing natural killer cell dysfunction and plasticity in cell-based cancer therapeutics. Cancers 15, 1743 (2023).
Laskowski, T. J., Biederstädt, A. & Rezvani, K. Natural killer cells in antitumour adoptive cell immunotherapy. Nat. Rev. Cancer 22, 557–575 (2022).
Ramírez-Labrada, A. et al. All About (NK cell-mediated) death in two acts and an unexpected encore: initiation, execution and activation of adaptive immunity. Front. Immunol. 13, 896228 (2022).
Böttcher, J. P. et al. NK cells stimulate recruitment of cDC1 into the tumor microenvironment promoting cancer immune control. Cell 172, 1022–1037 (2018).
Huntington, N. D., Cursons, J. & Rautela, J. The cancer-natural killer cell immunity cycle. Nat. Rev. Cancer 20, 437–454 (2020).
Pai, J. A. et al. Lineage tracing reveals clonal progenitors and long-term persistence of tumor-specific T cells during immune checkpoint blockade. Cancer Cell 41, 776–790 (2023).
Zheng, L. et al. Pan-cancer single-cell landscape of tumor-infiltrating T cells. Science 374, abe6474 (2021).
Oh, D. Y. et al. Intratumoral CD4+ T cells mediate anti-tumor cytotoxicity in human bladder cancer. Cell 181, 1612–1625(2020).
Zelenay, S. et al. Cyclooxygenase-dependent tumor growth through evasion of immunity. Cell 162, 1257–1270 (2015).
Cherkassky, L. et al. Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J. Clin. Invest. 126, 3130–3144 (2016).
Steele, M. M. et al. T cell egress via lymphatic vessels is tuned by antigen encounter and limits tumor control. Nat. Immunol. 24, 664–675 (2023). This study unveils the CXCR4–CXCL12 axis as an important target for enhancing intratumoral T cell retention in vivo.
Thacker, G. et al. Immature natural killer cells promote progression of triple-negative breast cancer. Sci. Transl. Med. 15, eabl4414 (2023). This study uncovers a ‘regulatory-like’ immature NK cell population that could be targeted to improve efficacy of immunotherapies against triple-negative breast cancer.
Nuñez, S. Y. et al. Human M2 macrophages limit NK cell effector functions through secretion of TGF-β and engagement of CD85j. J. Immunol. 200, 1008–1015 (2018).
Kloosterman, D. J. & Akkari, L. Macrophages at the interface of the co-evolving cancer ecosystem. Cell 186, 1627–1651 (2023).
Reinfeld, B. I. et al. Cell-programmed nutrient partitioning in the tumour microenvironment. Nature 593, 282–288 (2021).
Zalfa, C. & Paust, S. Natural killer cell interactions with myeloid derived suppressor cells in the tumor microenvironment and implications for cancer immunotherapy. Front. Immunol. 12, 633205 (2021).
Eschweiler, S. et al. Intratumoral follicular regulatory T cells curtail anti-PD-1 treatment efficacy. Nat. Immunol. 22, 1052–1063 (2021).
Maalej, K. M. et al. CAR-cell therapy in the era of solid tumor treatment: current challenges and emerging therapeutic advances. Mol. Cancer 22, 20 (2023).
Tai, L. H., Zhang, J. & Auer, R. C. Preventing surgery-induced NK cell dysfunction and cancer metastases with influenza vaccination. Oncoimmunology 2, e26618 (2013).
Liu, E. et al. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N. Engl. J. Med. 382, 545–553 (2020).
Xiao, X. et al. Mechanisms of cytokine release syndrome and neurotoxicity of CAR T-cell therapy and associated prevention and management strategies. J. Exp. Clin. Cancer Res. 40, 367 (2021).
Zhang, X. et al. Cytokine release syndrome after modified CAR-NK therapy in an advanced non-small cell lung cancer patient: a case report. Cell Transplant. 31, 9636897221094244 (2022).
Klingemann, H. Are natural killer cells superior CAR drivers? Oncoimmunology 3, e28147 (2014).
Paul, S. & Lal, G. The molecular mechanism of natural killer cells function and its importance in cancer immunotherapy. Front. Immunol. 8, 1124 (2017).
Whang, M. et al. Large-scale expansion and engineering of human NK cells sourced from peripheral blood versus umbilical cord blood. J. Immunother. Cancer 10, A401 (2022).
Min, B. et al. Optimization of large-scale expansion and cryopreservation of human natural killer cells for anti-tumor therapy. Immune Netw. 18, e31 (2018).
Davenport, A. J. et al. Chimeric antigen receptor T cells form nonclassical and potent immune synapses driving rapid cytotoxicity. Proc. Natl Acad. Sci. USA 115, E2068–E2076 (2018). This study identifies an immature immunological synapse formed by CAR molecules in CAR-T cells as compared to canonical TCR.
Monks, C. R., Freiberg, B. A., Kupfer, H., Sciaky, N. & Kupfer, A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature 395, 82–86 (1998).
Watanabe, K., Kuramitsu, S., Posey, A. D. Jr. & June, C. H. Expanding the therapeutic window for CAR T cell therapy in solid tumors: the knowns and unknowns of CAR T cell biology. Front. Immunol. 9, 2486 (2018).
Al-Aghbar, M. A., Jainarayanan, A. K., Dustin, M. L. & Roffler, S. R. The interplay between membrane topology and mechanical forces in regulating T cell receptor activity. Commun. Biol. 5, 40 (2022).
Somersalo, K. et al. Cytotoxic T lymphocytes form an antigen-independent ring junction. J. Clin. Invest. 113, 49–57 (2004).
Potter, T. A., Grebe, K., Freiberg, B. & Kupfer, A. Formation of supramolecular activation clusters on fresh ex vivo CD8+ T cells after engagement of the T cell antigen receptor and CD8 by antigen-presenting cells. Proc. Natl Acad. Sci. USA 98, 12624–12629 (2001).
Stinchcombe, J. C., Bossi, G., Booth, S. & Griffiths, G. M. The immunological synapse of CTL contains a secretory domain and membrane bridges. Immunity 15, 751–761 (2001).
Orange, J. S. Formation and function of the lytic NK-cell immunological synapse. Nat. Rev. Immunol. 8, 713–725 (2008).
Schmied, L. et al. SHP-1 localization to the activating immune synapse promotes NK cell tolerance in MHC class I deficiency. Sci. Signal. 16, eabq0752 (2023).
Davis, D. M. & Dustin, M. L. What is the importance of the immunological synapse? Trends Immunol. 25, 323–327 (2004).
Mukherjee, M., Mace, E. M., Carisey, A. F., Ahmed, N. & Orange, J. S. Quantitative imaging approaches to study the CAR immunological synapse. Mol. Ther. 25, 1757–1768 (2017).
Jenkins, M. R. et al. Failed CTL/NK cell killing and cytokine hypersecretion are directly linked through prolonged synapse time. J. Exp. Med. 212, 307–317 (2015).
Masilamani, M., Nguyen, C., Kabat, J., Borrego, F. & Coligan, J. E. CD94/NKG2A inhibits NK cell activation by disrupting the actin network at the immunological synapse. J. Immunol. 177, 3590–3596 (2006).
Williams, G. S. et al. Membranous structures transfer cell surface proteins across NK cell immune synapses. Traffic 8, 1190–1204 (2007).
McCann, F. E. et al. The size of the synaptic cleft and distinct distributions of filamentous actin, ezrin, CD43, and CD45 at activating and inhibitory human NK cell immune synapses. J. Immunol. 170, 2862–2870 (2003).
Zheng, X. et al. Tumors evade immune cytotoxicity by altering the surface topology of NK cells. Nat. Immunol. 24, 802–813 (2023). This study introduces a mechanism of tumor evasion in which tumor cells alter sphingomyelin content on intratumoral NK cells, leading to perturbed membrane protrusions and decreased cytotoxicity.
Chockley, P. J., Ibanez-Vega, J., Krenciute, G., Talbot, L. J. & Gottschalk, S. Synapse-tuned CARs enhance immune cell anti-tumor activity. Nat. Biotechnol. https://doi.org/10.1038/s41587-41022-01650-41582 (2023). This study introduces an approach by which tuning the synapse of CAR T or CAR NK cells could enhance their efficacy and intratumoral survival against solid tumors.
Lin, J. & Weiss, A. The tyrosine phosphatase CD148 is excluded from the immunologic synapse and down-regulates prolonged T cell signaling. J. Cell Biol. 162, 673–682 (2003).
Anikeeva, N. et al. Efficient killing of tumor cells by CAR-T cells requires greater number of engaged CARs than TCRs. J. Biol. Chem. 297, 101033 (2021).
Labanieh, L. & Mackall, C. L. CAR immune cells: design principles, resistance and the next generation. Nature 614, 635–648 (2023).
Kershaw, M. H. et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin. Cancer Res. 12, 6106–6115 (2006).
Tian, Y., Li, Y., Shao, Y. & Zhang, Y. Gene modification strategies for next-generation CAR T cells against solid cancers. J. Hematol. Oncol. 13, 54 (2020).
Kagoya, Y. et al. A novel chimeric antigen receptor containing a JAK-STAT signaling domain mediates superior antitumor effects. Nat. Med. 24, 352–359 (2018).
Dagher, O. K., Schwab, R. D., Brookens, S. K. & Posey, A. D. Jr. Advances in cancer immunotherapies. Cell 186, 1814–1814 (2023).
Curio, S., Jonsson, G. & Marinović, S. A summary of current NKG2D-based CAR clinical trials. Immunother. Adv. 1, ltab018 (2021).
Maali, A. et al. Nanobodies in cell-mediated immunotherapy: on the road to fight cancer. Front. Immunol. 14, 1012841 (2023).
Lin, H., Cheng, J., Mu, W., Zhou, J. & Zhu, L. Advances in universal CAR-T cell therapy. Front. Immunol. 12, 744823 (2021).
Wang, B. et al. Generation of hypoimmunogenic T cells from genetically engineered allogeneic human induced pluripotent stem cells. Nat. Biomed. Eng. 5, 429–440 (2021).
Doan, A. & Pulsipher, M.A. Hypogammaglobulinemia due to CAR T cell therapy. Pediatr. Blood Cancer 65, e26914 (2018).
Tousley, A. M. et al. Co-opting signalling molecules enables logic-gated control of CAR T cells. Nature 615, 507–516 (2023). This study introduces a gated-CAR platform in whichreplacing CD3ζ with downstream intracellular molecules such as SLP-76 and LAT enhanced the antitumor activity and mitigated on-target off-tumor toxicities.
Garrison, B. S. et al. FLT3 OR CD33 NOT EMCN logic gated CAR-NK cell therapy (SENTI-202) for precise targeting of AML. Blood 138, 2799 (2021).
Zhang, X., Zhu, L., Zhang, H., Chen, S. & Xiao, Y. CAR-T cell therapy in hematological malignancies: current opportunities and challenges. Front. Immunol. 13, 927153 (2022).
Sayitoglu, E. C. et al. Boosting natural killer cell-mediated targeting of sarcoma through DNAM-1 and NKG2D. Front. Immunol. 11, 40 (2020).
Ruppel, K. E., Fricke, S., Köhl, U. & Schmiedel, D. Taking Lessons from CAR-T cells and going beyond: tailoring design and signaling for CAR-NK cells in cancer therapy. Front. Immunol. 13, 822298 (2022).
Wrona, E., Borowiec, M. & Potemski, P. CAR-NK cells in the treatment of solid tumors. Int. J. Mol. Sci. 22, 5899 (2021).
Kloss, C. C. et al. Dominant-negative TGF-β receptor enhances PSMA-targeted human CAR T cell proliferation and augments prostate cancer eradication. Mol. Ther. 26, 1855–1866 (2018).
Chaudhry, K. et al. Co-transducing B7H3 CAR-NK cells with the DNR preserves their cytolytic function against GBM in the presence of exogenous TGF-β. Mol. Ther. Methods Clin. Dev. 27, 415–430 (2022).
Liu, X. et al. A novel dominant-negative PD-1 armored anti-CD19 CAR T cell is safe and effective against refractory/relapsed B cell lymphoma. Transl. Oncol. 14, 101085 (2021).
Liu, B. et al. Bifunctional TGF-β trap/IL-15 protein complex elicits potent NK cell and CD8+ T cell immunity against solid tumors. Mol. Ther. 29, 2949–2962 (2021).
Jung, I. Y. et al. CRISPR/Cas9-mediated knockout of DGK improves antitumor activities of human T cells. Cancer Res. 78, 4692–4703 (2018).
Aspuria, P. J. et al. An orthogonal IL-2 and IL-2Rβ system drives persistence and activation of CAR T cells and clearance of bulky lymphoma. Sci. Transl. Med. 13, eabg7565 (2021).
Zhang, Q. et al. A human orthogonal IL-2 and IL-2Rβ system enhances CAR T cell expansion and antitumor activity in a murine model of leukemia. Sci. Transl. Med. 13, eabg6986 (2021).
Thomas, S. & Abken, H. CAR T cell therapy becomes CHIC: ‘cytokine help intensified CAR’ T cells. Front. Immunol. 13, 1090959 (2022).
Ma, R. et al. An oncolytic virus expressing IL15/IL15Rα combined with off-the-shelf EGFR-CAR NK cells targets glioblastoma. Cancer Res. 81, 3635–3648 (2021).
Imai, C., Iwamoto, S. & Campana, D. Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood 106, 376–383 (2005).
Denman, C. J. et al. Membrane-bound IL-21 promotes sustained ex vivo proliferation of human natural killer cells. PLoS ONE 7, e30264 (2012).
Shum, T. et al. Constitutive signaling from an engineered IL7 receptor promotes durable tumor elimination by tumor-redirected T cells. Cancer Discov. 7, 1238–1247 (2017).
Adachi, K. et al. IL-7 and CCL19 expression in CAR-T cells improves immune cell infiltration and CAR-T cell survival in the tumor. Nat. Biotechnol. 36, 346–351 (2018).
Chen, Y. et al. Eradication of neuroblastoma by T cells redirected with an optimized GD2-specific chimeric antigen receptor and interleukin-15. Clin. Cancer Res. 25, 2915–2924 (2019).
Romee, R. et al. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci. Transl. Med. 8, 357ra123 (2016).
Wang, J. et al. Multispecific targeting of glioblastoma with tumor microenvironment-responsive multifunctional engineered NK cells. Proc. Natl Acad. Sci. USA 118, e2107507118 (2021).
Hegde, M. et al. Tandem CAR T cells targeting HER2 and IL13Rα2 mitigate tumor antigen escape. J. Clin. Invest. 126, 3036–3052 (2016).
Bielamowicz, K. et al. Trivalent CAR T cells overcome interpatient antigenic variability in glioblastoma. Neuro. Oncol. 20, 506–518 (2018).
Schmidts, A. et al. Tandem chimeric antigen receptor (CAR) T cells targeting EGFRvIII and IL-13Rα2 are effective against heterogeneous glioblastoma. Neurooncol. Adv. 5, vdac185 (2023).
Caruana, I. et al. Heparanase promotes tumor infiltration and antitumor activity of CAR-redirected T lymphocytes. Nat. Med. 21, 524–529 (2015).
Jin, L. et al. CXCR1- or CXCR2-modified CAR T cells co-opt IL-8 for maximal antitumor efficacy in solid tumors. Nat. Commun. 10, 4016 (2019).
Bughda, R., Dimou, P., D’Souza, R. R. & Klampatsa, A. Fibroblast activation protein (FAP)-targeted CAR-T cells: launching an attack on tumor stroma. Immunotargets Ther. 10, 313–323 (2021).
Weiner, L. M. inventor. Fibroblast activation protein modulation to alter immune cell migration and tumor infiltration. US patent application no. 22/165,019 (2022).
Storti, P. et al. Novel approaches to improve myeloma cell killing by monoclonal antibodies. J. Clin. Med. 9, 2864 (2020).
Rataj, F. et al. High-affinity CD16-polymorphism and Fc-engineered antibodies enable activity of CD16-chimeric antigen receptor-modified T cells for cancer therapy. Br. J. Cancer 120, 79–87 (2019).
Capuano, C. et al. Harnessing CD16-mediated NK cell functions to enhance therapeutic efficacy of tumor-targeting mAbs. Cancers 13, 2500 (2021).
Hsu, J. et al. Contribution of NK cells to immunotherapy mediated by PD-1/PD-L1 blockade. J. Clin. Invest. 128, 4654–4668 (2018).
Sharma, P. et al. Immune checkpoint therapy-current perspectives and future directions. Cell 186, 1652–1669 (2023).
Le, R. Q. et al. FDA approval summary: tocilizumab for treatment of chimeric antigen receptor T cell-induced severe or life-threatening cytokine release syndrome. Oncologist 23, 943–947 (2018).
Xing, J. et al. BiHC, a T-cell-engaging bispecific recombinant antibody, has potent cytotoxic activity against Her2 tumor cells. Transl. Oncol. 10, 780–785 (2017).
Goebeler, M. E. & Bargou, R. Blinatumomab: a CD19/CD3 bispecific T cell engager (BiTE) with unique anti-tumor efficacy. Leuk. Lymphoma 57, 1021–1032 (2016).
van Faassen, H. et al. Incorporation of a novel CD16-specific single-domain antibody into multispecific natural killer cell engagers with potent ADCC. Mol. Pharm. 18, 2375–2384 (2021).
Arvindam, U. S. et al. A trispecific killer engager molecule against CLEC12A effectively induces NK-cell mediated killing of AML cells. Leukemia 35, 1586–1596 (2021).
Gandhi, A. K. et al. Immunomodulatory agents lenalidomide and pomalidomide co-stimulate T cells by inducing degradation of T cell repressors Ikaros and Aiolos via modulation of the E3 ubiquitin ligase complex CRL4(CRBN.). Br. J. Haematol. 164, 811–821 (2014).
Wang, Z. et al. Lenalidomide enhances CAR-T cell activity against solid tumor cells. Cell Transplant. 29, 963689720920825 (2020).
Benson, D. M. Jr. et al. A phase I trial of the anti-KIR antibody IPH2101 and lenalidomide in patients with relapsed/refractory multiple myeloma. Clin. Cancer Res. 21, 4055–4061 (2015).
Park, A. K. et al. Effective combination immunotherapy using oncolytic viruses to deliver CAR targets to solid tumors. Sci. Transl. Med. 12, eaaz1863 (2020).
Liu, D. D. et al. Umbilical cord blood: a promising source for allogeneic CAR-T cells. Front. Oncol. 12, 944248 (2022).
Motwani, K. et al. Human regulatory T cells from umbilical cord blood display increased repertoire diversity and lineage stability relative to adult peripheral blood. Front. Immunol. 11, 611 (2020).
Zhu, X., Li, Q. & Zhu, X. Mechanisms of CAR T cell exhaustion and current counteraction strategies. Front. Cell Dev. Biol. 10, 1034257 (2022).
Arpinati, L. & Scherz-Shouval, R. From gatekeepers to providers: regulation of immune functions by cancer-associated fibroblasts. Trends Cancer 9, 421–443 (2023).
Finck, A. V., Blanchard, T., Roselle, C. P., Golinelli, G. & June, C. H. Engineered cellular immunotherapies in cancer and beyond. Nat. Med. 28, 678–689 (2022).
Brookens, S. K. & Posey, A. D. Jr. Chimeric antigen receptor T-cell therapy: current perspective on T cell-intrinsic, T cell-extrinsic, and therapeutic limitations. Cancer J. 29, 28–33 (2023).
Nayar, S., Dasgupta, P. & Galustian, C. Extending the lifespan and efficacies of immune cells used in adoptive transfer for cancer immunotherapies—a review. Oncoimmunology 4, e1002720 (2015).
Mark, C. et al. Cryopreservation impairs 3-D migration and cytotoxicity of natural killer cells. Nat. Commun. 11, 5224 (2020).
Yao, X. & Matosevic, S. Cryopreservation of NK and T cells without DMSO for adoptive cell-based immunotherapy. BioDrugs 35, 529–545 (2021).
Judge, S. J., Murphy, W. J. & Canter, R. J. Characterizing the dysfunctional NK cell: assessing the clinical relevance of exhaustion, anergy, and senescence. Front. Cell. Infect. Microbiol. 10, 49 (2020).
Heinrich, B. et al. The tumour microenvironment shapes innate lymphoid cells in patients with hepatocellular carcinoma. Gut 71, 1161–1175 (2022).
Zhang, W., Zhao, Z. & Li, F. Natural killer cell dysfunction in cancer and new strategies to utilize NK cell potential for cancer immunotherapy. Mol. Immunol. 144, 58–70 (2022).
El-Mayta, R., Zhang, Z., Hamilton, A. G. & Mitchell, M. J. Delivery technologies to engineer natural killer cells for cancer immunotherapy. Cancer Gene Ther. 28, 947–959 (2021).
Colamartino, A. B. L. et al. Efficient and robust NK-cell transduction with baboon envelope pseudotyped lentivector. Front. Immunol. 10, 2873 (2019).
Ojeda, P. Biological engineering of natural killer cells for cellular therapy against cancer. Master’s thesis, Harvard Medical School (2020).
Balke-Want, H. et al. Non-viral chimeric antigen receptor (CAR) T cells going viral. Immunooncol. Technol. 18, 100375 (2023).
Moretti, A. et al. The past, present, and future of non-viral CAR T cells. Front. Immunol. 13, 867013 (2022).
Bozza, M. et al. A nonviral, nonintegrating DNA nanovector platform for the safe, rapid, and persistent manufacture of recombinant T cells. Sci. Adv. 7, eabf1333 (2021).
Stadtmauer, E. A. et al. CRISPR-engineered T cells in patients with refractory cancer. Science 367, eaba7365 (2020).
Georgiadis, C. et al. Base-edited CAR T cells for combinational therapy against T cell malignancies. Leukemia 35, 3466–3481 (2021).
Tang, X. et al. Magnetic-acoustic sequentially actuated CAR T cell microrobots for precision navigation and in situ antitumor immunoactivation. Adv. Mater. 35, e2211509 (2023). This study uses biomedical engineering strategies that enable the use of CAR T cells as live microrobots that could be targeted and activated in situ.
Acknowledgements
A.D.P. is supported by funding from the V Foundation, Lustgarten Foundation and Veterans Affairs (I01 BX006247).
Author information
Authors and Affiliations
Contributions
O.K.D. and A.D.P. contributed to the writing and editing of the manuscript. O.K.D. designed the figures.
Corresponding authors
Ethics declarations
Competing interests
A.D.P. is an inventor on patents related to CAR T cell therapies. The other authors declare no competing interests.
Peer review
Peer review information
Nature Immunology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Nick Bernard, in collaboration with the Nature Immunology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dagher, O.K., Posey, A.D. Forks in the road for CAR T and CAR NK cell cancer therapies. Nat Immunol 24, 1994–2007 (2023). https://doi.org/10.1038/s41590-023-01659-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41590-023-01659-y
This article is cited by
-
In situ genetic engineering of host T-cells based on acellular scaffold strategy: a big but also small step for solid tumor immunotherapy
Military Medical Research (2024)
-
Combinational delivery of TLR4 and TLR7/8 agonist enhanced the therapeutic efficacy of immune checkpoint inhibitors to colon tumor
Molecular and Cellular Biochemistry (2024)