Abstract
Tau is an intrinsically disordered microtubule-associated protein (MAP) implicated in neurodegenerative disease. On microtubules, tau molecules segregate into two kinetically distinct phases, consisting of either independently diffusing molecules or interacting molecules that form cohesive ‘envelopes’ around microtubules. Envelopes differentially regulate lattice accessibility for other MAPs, but the mechanism of envelope formation remains unclear. Here we find that tau envelopes form cooperatively, locally altering the spacing of tubulin dimers within the microtubule lattice. Envelope formation compacted the underlying lattice, whereas lattice extension induced tau envelope disassembly. Investigating other members of the tau family, we find that MAP2 similarly forms envelopes governed by lattice spacing, whereas MAP4 cannot. Envelopes differentially biased motor protein movement, suggesting that tau family members could spatially divide the microtubule surface into functionally distinct regions. We conclude that the interdependent allostery between lattice spacing and cooperative envelope formation provides the molecular basis for spatial regulation of microtubule-based processes by tau and MAP2.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Source data files for all figures and Supplementary figures, are available with this manuscript. Source data are provided with this paper.
References
Alushin, G. M. et al. High-resolution microtubule structures reveal the structural transitions in αβ-tubulin upon GTP hydrolysis. Cell 157, 1117–1129 (2014).
Hyman, A. A., Salser, S., Drechsel, D. N., Unwin, N. & Mitchison, T. J. Role of GTP hydrolysis in microtubule dynamics: information from a slowly hydrolyzable analogue, GMPCPP. Mol. Biol. Cell 3, 1155–1167 (1992).
Dı́az, J. F., Barasoain, I. & Andreu, J. M. Fast kinetics of taxol binding to microtubules: effects of solution variables and microtubule-associated proteins. J. Biol. Chem. 278, 8407–8419 (2003).
Peet, D. R., Burroughs, N. J. & Cross, R. A. Kinesin expands and stabilizes the GDP-microtubule lattice. Nat. Nanotechnol. 13, 386–391 (2018).
Zhang, R., LaFrance, B. & Nogales, E. Separating the effects of nucleotide and EB binding on microtubule structure. Proc. Natl Acad. Sci. 115, E6191–E6200 (2018).
Shima, T. et al. Kinesin-binding–triggered conformation switching of microtubules contributes to polarized transport. J. Cell Biol. 217, 4164–4183 (2018).
Maurer, S. P., Bieling, P., Cope, J., Hoenger, A. & Surrey, T. GTPγS microtubules mimic the growing microtubule end structure recognized by end-binding proteins (EBs). Proc. Natl Acad. Sci. 108, 3988–3993 (2011).
Zanic, M., Stear, J. H., Hyman, A. A. & Howard, J. EB1 recognizes the nucleotide state of tubulin in the microtubule lattice. PLoS One 4, e7585 (2009).
Tan, R. et al. Microtubules gate tau condensation to spatially regulate microtubule functions. Nat. Cell Biol. 21, 1078–1085 (2019).
Castle, B. T., McKibben, K. M., Rhoades, E. & Odde, D. J. Tau avoids the GTP cap at growing microtubule plus-ends. iScience 23, 101782 (2020).
Guedes-Dias, P. et al. Kinesin-3 responds to local microtubule dynamics to target synaptic cargo delivery to the presynapse. Curr. Biol. 29, 268–282 (2019).
Dehmelt, L. & Halpain, S. The MAP2/Tau family of microtubule-associated proteins. Genome Biol. 6, 1–10 (2005).
Sündermann, F., Fernandez, M. & Morgan, R. An evolutionary roadmap to the microtubule-associated protein MAP Tau. BMC Genom. 17, 264 (2016).
Götz, J., Halliday, G. & Nisbet, R. M. Molecular pathogenesis of the tauopathies. Annu. Rev. Pathol. Mech. Dis. 14, 239–261 (2019).
Li, L., Zhang, Q., Lei, X., Huang, Y. & Hu, J. MAP4 as a new candidate in cardiovascular disease. Front. Physiol. 11, 1044 (2020).
Monroy, B. Y. et al. A combinatorial MAP code dictates polarized microtubule transport. Dev. Cell 53, 60–72 (2020).
Seitz, A. et al. Single-molecule investigation of the interference between kinesin, tau and MAP2c. EMBO J. 21, 4896–4905 (2002).
Semenova, I. et al. Regulation of microtubule-based transport by MAP4. Mol. Biol. Cell 25, 3119–3132 (2014).
Samora, C. P. et al. MAP4 and CLASP1 operate as a safety mechanism to maintain a stable spindle position in mitosis. Nat. Cell Biol. 13, 1040–1050 (2011).
Karasmanis, E. P. et al. Polarity of neuronal membrane traffic requires sorting of kinesin motor cargo during entry into dendrites by a microtubule-associated septin. Dev. Cell 46, 204–218 (2018).
Bulinski, J. C., McGraw, T. E., Gruber, D., Lan Nguyen, H. & Sheetz, M. P. Overexpression of MAP4 inhibits organelle motility and trafficking in vivo. J. Cell Sci. 110, 3055–3064 (1997).
Hernández-Vega, A. et al. Local nucleation of microtubule bundles through tubulin concentration into a condensed Tau phase. Cell Rep. 20, 2304–2312 (2017).
Zhang, X. et al. The proline-rich domain promotes Tau liquid–liquid phase separation in cells. J. Cell Biol. 219, e202006054 (2020).
Zhang, X. et al. RNA stores tau reversibly in complex coacervates. PLoS Biol. 15, e2002183 (2017).
Iqbal, K., Liu, F. & Gong, C.-X. Tau and neurodegenerative disease: the story so far. Nat. Rev. Neurol. 12, 15–27 (2016).
Dixit, R., Ross, J. L., Goldman, Y. E. & Holzbaur, E. L. F. Differential regulation of dynein and kinesin motor proteins by tau. Science 319, 1086–1089 (2008).
McVicker, D. P., Hoeprich, G. J., Thompson, A. R. & Berger, C. L. Tau interconverts between diffusive and stable populations on the microtubule surface in an isoform and lattice specific manner. Cytoskeleton 71, 184–194 (2014).
Siahaan, V. et al. Kinetically distinct phases of tau on microtubules regulate kinesin motors and severing enzymes. Nat. Cell Biol. 21, 1086–1092 (2019).
Chaudhary, A. R., Berger, F., Berger, C. L. & Hendricks, A. G. Tau directs intracellular trafficking by regulating the forces exerted by kinesin and dynein teams. Traffic 19, 111–121 (2018).
Diaz, J. F. & Andreu, J. M. Assembly of purified GDP-tubulin into microtubules induced by taxol and taxotere: reversibility, ligand stoichiometry, and competition. Biochemistry 32, 2747–2755 (1993).
Kar, S., Fan, J., Smith, M. J., Goedert, M. & Amos, L. A. Repeat motifs of tau bind to the insides of microtubules in the absence of taxol. EMBO J. 22, 70–77 (2003).
Lin, Y. et al. Toxic PR poly-dipeptides encoded by the C9orf72 repeat expansion target LC domain polymers. Cell 167, 789–802.e12 (2016).
Samsonov, A., Yu, J.-Z., Rasenick, M. & Popov, S. V. Tau interaction with microtubules in vivo. J. Cell Sci. 117, 6129–6141 (2004).
Balabanian, L., Berger, C. L. & Hendricks, A. G. Acetylated microtubules are preferentially bundled leading to enhanced kinesin-1 motility. Biophys. J. 113, 1551–1560 (2017).
Ettinger, A., van Haren, J., Ribeiro, S. A. & Wittmann, T. Doublecortin is excluded from growing microtubule ends and recognizes the GDP-microtubule lattice. Curr. Biol. 26, 1549–1555 (2016).
McKenney, R. J., Huynh, W., Vale, R. D. & Sirajuddin, M. Tyrosination of α-tubulin controls the initiation of processive dynein–dynactin motility. EMBO J. 35, 1175–1185 (2016).
Lam, A. J. et al. A highly conserved 310 helix within the kinesin motor domain is critical for kinesin function and human health. Sci. Adv. 7, eabf1002.
Budaitis, B. G. et al. Pathogenic mutations in the kinesin-3 motor KIF1A diminish force generation and movement through allosteric mechanisms. J. Cell Biol. 220, e202004227 (2021).
Chiba, K. et al. Disease-associated mutations hyperactivate KIF1A motility and anterograde axonal transport of synaptic vesicle precursors. Proc. Natl Acad. Sci. 116, 18429–18434 (2019).
Boyle, L. et al. Genotype and defects in microtubule-based motility correlate with clinical severity in KIF1A-associated neurological disorder. Hum. Genet. Genomics Adv. 2, 100026 (2021).
Kellogg, E. H. et al. Near-atomic model of microtubule–tau interactions. Science 360, 1242–1246 (2018).
Wijeratne, S. S., Fiorenza, S. A., Subramanian, R. & Betterton, M. D. Motor guidance by long-range communication through the microtubule highway. Preprint at bioRxiv https://doi.org/10.1101/2020.12.23.424221 (2020).
Kim, T. & Rice, L. M. Long-range, through-lattice coupling improves predictions of microtubule catastrophe. Mol. Biol. Cell 30, 1451–1462 (2019).
Shigematsu, H. et al. Structural insight into microtubule stabilization and kinesin inhibition by Tau family MAPs. J. Cell Biol. 217, 4155–4163 (2018).
Gu, Y., Oyama, F. & Ihara, Y. Tau is widely expressed in rat tissues. J. Neurochem. 67, 1235–1244 (1996).
Shults, N. V. et al. Tau protein in lung smooth muscle cells. J. Respir. 1, 30–39 (2020).
Howes, S. C. et al. Structural differences between yeast and mammalian microtubules revealed by cryo-EM. J. Cell Biol. 216, 2669–2677 (2017).
Chaaban, S. et al. The structure and dynamics of C. elegans tubulin reveals the mechanistic basis of microtubule growth. Dev. Cell 47, 191–204 (2018).
Triclin, S. et al. Self-repair protects microtubules from destruction by molecular motors. Nat. Mater. 20, 883–891 (2021).
Soppina, V. & Verhey, K. J. The family-specific K-loop influences the microtubule on-rate but not the superprocessivity of kinesin-3 motors. Mol. Biol. Cell 25, 2161–2170 (2014).
Henrichs, V. et al. Mitochondria-adaptor TRAK1 promotes kinesin-1 driven transport in crowded environments. Nat. Commun. 2020 111 11, 1–13 (2020).
Gell, C. et al. Purification of tubulin from porcine brain. Methods Mol. Biol. 777, 15–28 (2011).
Tan, R., Foster, P. J., Needleman, D. J. & McKenney, R. J. Cooperative accumulation of dynein–dynactin at microtubule minus-ends drives microtubule network reorganization. Dev. Cell 44, 233–247 (2018).
McKenney, R. J., Huynh, W., Tanenbaum, M. E., Bhabha, G. & Vale, R. D. Activation of cytoplasmic dynein motility by dynactin-cargo adapter complexes. Science 345, 337–341 (2014).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Zivanov, J. et al. New tools for automated high-resolution cryo-EM structure determination in RELION-3. eLife 7, e42166 (2018).
Chrétien, D., Kenney, J. M., Fuller, S. D. & Wade, R. H. Determination of microtubule polarity by cryo-electron microscopy. Structure 4, 1031–1040 (1996).
Acknowledgements
The authors thank members of the MOM lab for feedback and discussion during the project and the Protein Facility of MPI-CBG and V. Váňová for technical support. We also thank A. Carter for critical reading of the manuscript, O. Kučera for establishing the optical tweezers assay and J. Sabó for support in obtaining imaging data. This work was supported by Czech Science Foundation grant 19–27477X to Z.L. and L.L. and grant 20-04068S to M.B. We acknowledge the financial support from the Charles University Grant Agency (GAUK no. 373821 to V.S.) and the project ‘Grant Schemes at CU’ (reg. no. CZ.02.2.69/0.0/0.0/19_073/0016935) to T.H. and V.S., grants 1R35GM124889 to R.J.M. and 1R35GM133688 to K.M.O.M. K.M.O.M. is also supported by the Pew Charitable Trusts grant A19-0406. We acknowledge the institutional support from the CAS (RVO: 86652036), CMS supported by MEYS CR (LM2015043), and the Imaging Methods Core Facility at BIOCEV, an institution supported by the MEYS CR (Large RI Project LM2018129 Czech-BioImaging) and ERDF (project no. CZ.02.1.01/0.0/ 0.0/16_013/0001775) for their support in obtaining imaging data presented in this paper.
Author information
Authors and Affiliations
Contributions
The manuscript was conceptualized by R.T., K.M.O.M., R.J.M., M.B., and Z.L.; methods were developed by V.S., T.H., L.L., S.E.L., R.J.M., M.B., and Z.L.; recombinant proteins were generated by V.S., R.T., T.T., and M.D.; TIRF experiments were performed by V.S., R.T., and R.J.M.; optical tweezers experiments by V.S.; cryo-EM experiments by S.E.L.; live-cell experiments by T.H.; data were formally analyzed by V.S., R.T., T.H., S.E.L., K.M.O.M., and R.J.M.; the manuscript was written by V.S., L.L., K.M.O.M., R.J.M., M.B., Z.L., with reviewing and editing by T.T. and M.D.; the project was supervised by K.M.O.M., R.J.M., M.B., and Z.L.; funding was acquired by V.S., T.H., L.L., K.M.O.M., R.J.M., M.B., and Z.L.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemical Biology thanks Arne Gennerich and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs 1–4, Supplementary Movie captions, Source data files, Supplementary Data files.
Supplementary Video 1
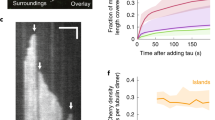
Video related to Fig. 1c. Structural changes in the microtubule lattice upon cooperative binding of tau. Time-lapse movie of tau-mCherry (green) envelopes growing on a taxol-lattice microtubule. Twenty nanomolar tau-mCherry is added to an initially bent microtubule. The microtubule straightens out when the tau envelope forms in the curved region. Scale bar, 2 µm.
Supplementary Video 2
Video related to Fig. 1e. Kinesin-1 walking on taxol- and GMPCPP-lattice microtubules in presence of elevated concentrations of tau. Time-lapse movie of kinesin-1 (magenta) stepping on taxol- and GMPCPP-lattice microtubules in the presence of elevated concentrations of tau (green). Sixty nanomolar kinesin-1-GFP was added to a taxol-lattice microtubule (top) and a GMPCPP-lattice microtubule (bottom) in the presence of 600 nM tau-mCherry. Processive movement of kinesin-1 was detected over the entire length of GMPCPP-lattice microtubules, while no processive movement of kinesin-1 was detected on taxol-lattice microtubules, indicating the presence of tau envelopes on taxol-lattice microtubules, and the absence of tau envelopes on GMPCPP-lattice microtubules even at these elevated concentrations of tau. Scale bar, 2 µm.
Supplementary Video 3
Video related to Fig. 1g. Compaction of a speckled-labeled microtubule upon cooperative binding of tau. Time-lapse movie of a speckled microtubule after the addition of tau. 200 nM tau-eGFP (green) was added to a speckled-labeled taxol-lattice microtubule (speckles in white). Upon cooperative binding of tau, speckles can be seen moving closer to each other, indicating the compaction of the microtubule lattice. Scale bar: 2 µm
Supplementary Video 4
Video related to Fig. 1i. SiR-tubulin dissociation of microtubule lattice within tau envelopes regions. Time-lapse movie of 2 μM SiR-tubulin (magenta) on the microtubule lattice after the addition of 20 nM tau-mCherry (green). At the positions where tau envelopes grow on the microtubule lattice (left), the density of SiR-tubulin locally decreases (middle). Right, overlay. Scale bar, 2 µm.
Supplementary Video 5
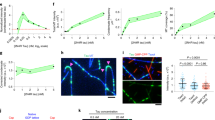
Video related to Fig. 2a. Local extension of GDP-lattice microtubule induces tau envelope disassembly in the presence of taxol. Time-lapse movie of 20 nM tau-mCherry (green) on a GMPCPP-capped GDP-lattice microtubule. At t = 0 s, tau is removed from the solution and microtubules are bent using hydrodynamic flow. During the hydrodynamic flow, imaging buffer with 10 μM taxol is added to the measurement chamber. In the presence of taxol, tau envelopes disassemble from their boundaries as well as from the location of the initially bent position. Scale bar, 5 µm.
Supplementary Video 6
Video related to Fig. 2a. Local extension of GDP-lattice microtubule does not induce tau envelope disassembly in the absence of taxol. Time-lapse movie of 20 nM tau-mCherry (green) on GMPCPP-capped GDP-lattice microtubules. At t = 0 s, tau is removed from the solution and microtubules are bent using hydrodynamic flow. During the hydrodynamic flow, imaging buffer without taxol is added to the measurement chamber. In the absence of taxol, no tau envelope disassembly was observed and disruptions in the tau envelopes owing to bending the microtubule lattice were found to reform within a 200 s timeframe. Scale bar, 5 µm.
Supplementary Video 7
Video related to Fig. 2g. Global extension of the microtubule lattice in the optical tweezers induces faster envelope disassembly. Time-lapse movie of the disassembly of tau envelopes on a microtubule suspended between two beads and stretched using external force. Initially, a single taxol-lattice microtubule is suspended between two beads and 60 nM tau-mCherry is added to the measurement chamber. The microtubule is then globally extended by slowly moving the beads apart until a set force of 40 pN is measured. Tau is then removed from solution so that envelope disassembly is observed. Global extension of the microtubule lattice in the optical tweezers induces faster envelope disassembly. Scale bar, 3 µm.
Supplementary Video 8
Video related to Fig. 2i. In the absence of global extension of the microtubule lattice in the optical tweezers, tau envelopes disassemble slower. Time-lapse movie of the disassembly of tau envelopes on a microtubule attached to a single bead and relaxed in the absence of external force. Initially, a single taxol-lattice microtubule is attached to a bead and 60 nM tau-mCherry is added to the measurement chamber. Tau is then removed from solution so that envelope disassembly is observed. In the absence of global extension of the microtubule lattice in the optical tweezers, tau envelopes disassemble slower. Scale bar, 3 µm.
Supplementary Video 9
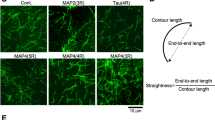
Video related to Fig. 4a. Tau dissociates from microtubules in vivo upon addition of taxol. Time-lapse movie of a U-2 OS cell expressing eGFP-tau (white) after the addition of 0.01 μM taxol. Tau signal can be seen gradually dissociating from the microtubules in vivo after the addition of taxol. Scale bar, 10 µm.
Supplementary Video 10
Video related to Fig. 4a. Tau does not dissociate from microtubules after the addition of DMSO. Time-lapse movie of a U-2 OS cell expressing eGFP-tau (white) after the addition of DMSO in the absence of taxol. Tau remains on the microtubules in vivo in the control experiment in the absence of taxol. Scale bar, 10 µm.
Supplementary Video 11
Video related to Fig. 4c. MAP4 does not dissociate from microtubules in vivo upon addition of taxol. Time-lapse movie of a U-2 OS cell expressing eGFP-MAP4 after the addition of 0.01 μM taxol. In contrast to tau, MAP4 remains on the microtubules in vivo after the addition of taxol. Scale bar, 10 µm.
Supplementary Video 12
Video related to Fig. 4c. MAP4 does not dissociate from microtubules in vivo upon addition of DMSO. Time-lapse movie of a U-2 OS cell expressing eGFP-MAP4 after the addition of DMSO in the absence of taxol. MAP4 remains on the microtubules in vivo in the control experiment in the absence of taxol. Scale bar, 10 µm.
Supplementary Video 13
Video related to Fig. 4e. Tau dissociates from microtubules in vivo strongly resembling tau envelope disassembly in vitro. Time-lapse movie of a single microtubule in a U-2 OS cell expressing eGFP-tau after the addition of 0.01 μM taxol at t = 0 min. During the dissociation of tau from the microtubules, fissions appear in the tau density that disassemble from their boundaries, closely resembling tau envelope disassembly in vitro. Scale bar, 2 µm.
Supplementary Video 14
Video related to Supplementary Figure 4d. Tau dissociates from microtubules in vivo strongly resembling tau envelope disassembly in vitro. Time-lapse movie of a second example of a single microtubule in a U-2 OS cell expressing eGFP-tau after the addition of 0.01 μM taxol at t = 0 min. During the dissociation of tau from the microtubules, fissions appear in the tau density that disassemble from their boundaries, closely resembling tau envelope disassembly in vitro. Scale bar, 1 µm.
Supplementary Video 15
Video related to Supplementary Figure 4d. Tau dissociates from microtubules in vivo strongly resembling tau envelope disassembly in vitro. Time-lapse movie of a third example of a single microtubule in a U-2 OS cell expressing eGFP-tau after the addition of 0.01 μM taxol at t = 0 min. During the dissociation of tau from the microtubules, fissions appear in the tau density that disassemble from their boundaries, closely resembling tau envelope disassembly in vitro. Scale bar, 1 µm.
Supplementary Data 1
Statistical Source Data for Supplementary Figure 1.
Supplementary Data 2
Statistical Source Data for Supplementary Figure 2.
Supplementary Data 3
Statistical Source Data for Supplementary Figure 3.
Supplementary Data 4
Statistical Source Data for Supplementary Figure 4.
Source data
Source Data Fig. 1
Statistical Source Data.
Source Data Fig. 2
Statistical Source Data.
Source Data Fig. 3
Statistical Source Data.
Source Data Fig. 3
Unprocessed gels.
Source Data Fig. 4
Statistical Source Data.
Source Data Fig. 5
Statistical Source Data.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Siahaan, V., Tan, R., Humhalova, T. et al. Microtubule lattice spacing governs cohesive envelope formation of tau family proteins. Nat Chem Biol 18, 1224–1235 (2022). https://doi.org/10.1038/s41589-022-01096-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-022-01096-2
This article is cited by
-
Taxol acts differently on different tubulin isotypes
Communications Biology (2023)