Abstract
Distinct tissue-specific mechanisms mediate insulin action in fasting and postprandial states. Previous genetic studies have largely focused on insulin resistance in the fasting state, where hepatic insulin action dominates. Here we studied genetic variants influencing insulin levels measured 2 h after a glucose challenge in >55,000 participants from three ancestry groups. We identified ten new loci (P < 5 × 10−8) not previously associated with postchallenge insulin resistance, eight of which were shown to share their genetic architecture with type 2 diabetes in colocalization analyses. We investigated candidate genes at a subset of associated loci in cultured cells and identified nine candidate genes newly implicated in the expression or trafficking of GLUT4, the key glucose transporter in postprandial glucose uptake in muscle and fat. By focusing on postprandial insulin resistance, we highlighted the mechanisms of action at type 2 diabetes loci that are not adequately captured by studies of fasting glycemic traits.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
GWAS summary statistics will be made available on the MAGIC investigators.
Website (https://magicinvestigators.org/downloads/) and GWAS catalog (https://www.ebi.ac.uk/gwas/home): GCST90267567, GCST90267568, GCST90267569, GCST90267570, GCST90267571, GCST90267572, GCST90267573, GCST90267574, GCST90267575, GCST90267576, GCST90267577 and GCST90267578.
Data from the Fenland cohort can be requested by bona fide researchers for specified scientific purposes via the study website (https://www.mrc-epid.cam.ac.uk/research/studies/fenland/information-for-researchers/). Data will either be shared through an institutional data-sharing agreement or arrangements will be made for analyses to be conducted remotely without the necessity for data transfer.
All data used in genetic risk score association analyses are available from the UK Biobank upon application (https://www.ukbiobank.ac.uk). All analyses in the UK Biobank in this manuscript were conducted under application 44448. Further details about the RISC study and data availability can be found here: http://www.egir.org/egirrisc/. The Genotype-Tissue Expression (GTEx) Project was supported by the Common Fund of the Office of the Director of the National Institutes of Health, and by NCI, NHGRI, NHLBI, NIDA, NIMH and NINDS. The data used for the analyses described in this manuscript can be obtained from the GTEx Portal (https://www.gtexportal.org/home/) and dbGaP accession number phs000424.v8.p2. Genome regulatory annotations from ENCODE (https://www.encodeproject.org/) and Roadmap Epigenomics Consortium (https://egg2.wustl.edu/roadmap/web_portal/) were explored via UCSC Genome Browser (http://genome.ucsc.edu). Published differentiated 3T3-L1 RNA-sequencing data used in this study are available from GEO accession GSE129957 (https://www.ncbi.nlm.nih.gov/geo/). Source data are provided with this paper.
Code availability
No previously unreported custom code or algorithm was used to generate results. The following software and packages were used for data analysis: METAL v.2011-03-25 (http://csg.sph.umich.edu/abecasis/Metal/download/), random-metal v.2017-07-24 (https://github.com/explodecomputer/random-metal), linkage disequilibrium score regression v.1.0.1 (https://github.com/bulik/ldsc), R v.3.6.0 and v.4.0.3 (https://www.r-project.org/). R packages coloc v.5.1.0 (https://cran.r-project.org/web/packages/coloc/).
Hyprcoloc v.1.0 (https://github.com/jrs95/hyprcoloc).
GCTA 1.26.0 (https://yanglab.westlake.edu.cn/software/gcta/#Overview). EasyQC v.17.8 (https://www.uni-regensburg.de/medizin/epidemiologie-praeventivmedizin/genetische-epidemiologie/software/index.html). Associated code and scripts used in this manuscript are available on GitHub: https://github.com/MRC-Epid/GWAS_postchallenge_insulin (https://zenodo.org/record/7805583#.ZC7C_exBxhE).
References
James, D. E., Stöckli, J. & Birnbaum, M. J. The aetiology and molecular landscape of insulin resistance. Nat. Rev. Mol. Cell Biol. 22, 751–771 (2021).
Defronzo, R. A. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 58, 773–795 (2009).
Chen, J. et al. The trans-ancestral genomic architecture of glycemic traits. Nat. Genet. 53, 840–860 (2021).
Scott, R. A. et al. Large-scale association analyses identify new loci influencing glycemic traits and provide insight into the underlying biological pathways. Nat. Genet. 44, 991–1005 (2012).
Lagou, V. et al. Sex-dimorphic genetic effects and novel loci for fasting glucose and insulin variability. Nat. Commun. 12, 1–18 (2021).
Taylor, R. et al. Direct assessment of liver glycogen storage by 13C nuclear magnetic resonance spectroscopy and regulation of glucose homeostasis after a mixed meal in normal subjects. J. Clin. Invest. 97, 126–132 (1996).
Jue, T., Rothman, D. L., Tavitian, B. A. & Shulman, R. G. Natural-abundance 13C NMR study of glycogen repletion in human liver and muscle. Proc. Natl Acad. Sci. USA 86, 1439–1442 (1989).
Petersen, M. C. & Shulman, G. I. Mechanisms of insulin action and insulin resistance. Physiol. Rev. 98, 2133 (2018).
Fischer, Y. et al. Insulin-induced recruitment of glucose transporter 4 (GLUT4) and GLUT1 in isolated rat cardiac myocytes. Evidence of the existence of different intracellular GLUT4 vesicle populations. J. Biol. Chem. 272, 7085–7092 (1997).
Goodyear, L. J. et al. Glucose ingestion causes GLUT4 translocation in human skeletal muscle. Diabetes 45, 1051–1056 (1996).
Kahn, B. B. Dietary regulation of glucose transporter gene expression: tissue specific effects in adipose cells and muscle. J. Nutr. 124, 1289S–1295S (1994).
Maianu, L., Keller, S. R. & Garvey, W. T. Adipocytes exhibit abnormal subcellular distribution and translocation of vesicles containing glucose transporter 4 and insulin-regulated aminopeptidase in type 2 diabetes mellitus: implications regarding defects in vesicle trafficking. J. Clin. Endocrinol. Metab. 86, 5450–5456 (2001).
Rothman, D. L. et al. Decreased muscle glucose transport/phosphorylation is an early defect in the pathogenesis of non-insulin-dependent diabetes mellitus. Proc. Natl Acad. Sci. USA 92, 983–987 (1995).
DeFronzo, R. A. & Tripathy, D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care 32, S157 (2009).
Sano, H. et al. Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J. Biol. Chem. 278, 14599–14602 (2003).
Dash, S. et al. A truncation mutation in TBC1D4 in a family with acanthosis nigricans and postprandial hyperinsulinemia. Proc. Natl Acad. Sci. USA 106, 9350–9355 (2009).
Grarup, N. et al. Identification of novel high-impact recessively inherited type 2 diabetes risk variants in the Greenlandic population. Diabetologia 61, 2005–2015 (2018).
Moltke, I. et al. A common Greenlandic TBC1D4 variant confers muscle insulin resistance and type 2 diabetes. Nature 512, 190–193 (2014).
Tam, C. S. et al. Defining insulin resistance from hyperinsulinemic-euglycemic clamps. Diabetes Care 35, 1605–1610 (2012).
Reinauer, H. et al. Determination of glucose turnover and glucose oxidation rates in man with stable isotope tracers. J. Clin. Chem. Clin. Biochem. 28, 505–512 (1990).
Muniyappa, R., Lee, S., Chen, H. & Quon, M. J. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am. J. Physiol. Endocrinol. Metab. 294, 15–26 (2008).
Stumvoll, M. et al. Use of the oral glucose tolerance test to assess insulin release and insulin sensitivity. Diabetes Care 23, 295–301 (2000).
Walford, G. A. et al. Genome-wide association study of the modified Stumvoll insulin sensitivity index identifies BCL2 and FAM19A2 as novel insulin sensitivity loci. Diabetes 65, 3200–3211 (2016).
Dimas, A. S. et al. Impact of type 2 diabetes susceptibility variants on quantitative glycemic traits reveals mechanistic heterogeneity. Diabetes 63, 2158–2171 (2014).
DeFronzo, R. A., Tobin, J. D. & Andres, R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am. J. Physiol. 237, E214–E223 (1979).
Vujkovic, M. et al. Discovery of 318 new risk loci for type 2 diabetes and related vascular outcomes among 1.4 million participants in a multi-ancestry meta-analysis. Nat. Genet. 52, 680–691 (2020).
Zhu, Y., Wang, L., Yin, Y. & Yang, E. Systematic analysis of gene expression patterns associated with postmortem interval in human tissues. Sci. Rep. 7, 5435 (2017).
Uhlén, M. et al. Proteomics. Tissue-based map of the human proteome. Science 347, 1260419 (2015).
Aguet, F. et al. The GTEx Consortium atlas of genetic regulatory effects across human tissues. Science 369, 1318–1330 (2020).
Lonsdale, J. et al. The Genotype-Tissue Expression (GTEx) project. Nat. Genet. 45, 580–585 (2013).
Kanai, F. et al. Insulin-stimulated GLUT4 translocation is relevant to the phosphorylation of IRS-1 and the activity of PI3-kinase. Biochem. Biophys. Res. Commun. 195, 762–768 (1993).
Keller, S. R., Scott, H. M., Mastick, C. C., Aebersold, R. & Lienhard, G. E. Cloning and characterization of a novel insulin-regulated membrane aminopeptidase from Glut4 vesicles. J. Biol. Chem. 270, 23612–23618 (1995).
Chi, N. W. & Lodish, H. F. Tankyrase is a Golgi-associated mitogen-activated protein kinase substrate that interacts with IRAP in GLUT4 vesicles. J. Biol. Chem. 275, 38437–38444 (2000).
Guo, H. L. et al. The Axin/TNKS complex interacts with KIF3A and is required for insulin-stimulated GLUT4 translocation. Cell Res. 22, 1246–1257 (2012).
Hook, S. C. et al. TBC1D1 interacting proteins, VPS13A and VPS13C, regulate GLUT4 homeostasis in C2C12 myotubes. Sci. Rep. 10, 17953 (2020).
Klip, A., McGraw, T. E. & James, D. E. Thirty sweet years of GLUT4. J. Biol. Chem. 294, 11369–11381 (2019).
Stenbit, A. E. et al. GLUT4 heterozygous knockout mice develop muscle insulin resistance and diabetes. Nat. Med. 3, 1096–1101 (1997).
Gual, P., Le Marchand-Brustel, Y. & Tanti, J. F. Positive and negative regulation of insulin signaling through IRS-1 phosphorylation. Biochimie 87, 99–109 (2005).
Barroso, I. Dominant negative mutations in human PPARγ associated with severe insulin resistance, diabetes mellitus and hypertension. Nature 402, 880–883 (1999).
Li, Q. et al. The protein phosphatase 1 complex is a direct target of AKT that links insulin signaling to hepatic glycogen deposition. Cell Rep. 28, 3406–3422 (2019).
Agius, L. Role of glycogen phosphorylase in liver glycogen metabolism. Mol. Asp. Med. 46, 34–45 (2015).
Yoon, M. S. et al. The role of mammalian target of rapamycin (mTOR) in insulin signaling. Nutrients 9, 1176 (2017).
Kuo, T. et al. Identification of C2CD4A as a human diabetes susceptibility gene with a role in β cell insulin secretion. Proc. Natl Acad. Sci. USA 116, 20033–20042 (2019).
Lyssenko, V. et al. Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat. Genet. 41, 82–88 (2009).
Huang, S. & Czech, M. P. The GLUT4 glucose transporter. Cell Metab. 5, 237–252 (2007).
Degrandmaison, J. et al. In vivo mapping of a GPCR interactome using knockin mice. Proc. Natl Acad. Sci. USA 117, 13105–13116 (2020).
Mani, M. et al. DRG2 knockdown induces Golgi fragmentation via GSK3β phosphorylation and microtubule stabilization. Biochim. Biophys. Acta Mol. Cell. Res. 1866, 1463–1474 (2019).
Mani, M. et al. Developmentally regulated GTP-binding protein 2 coordinates Rab5 activity and transferrin recycling. Mol. Biol. Cell 27, 334–348 (2016).
Gendre, D. et al. Conserved Arabidopsis ECHIDNA protein mediates trans-Golgi-network trafficking and cell elongation. Proc. Natl Acad. Sci. USA 108, 8048–8053 (2011).
Gonzales, P. A. et al. Large-scale proteomics and phosphoproteomics of urinary exosomes. J. Am. Soc. Nephrol. 20, 363–379 (2009).
Wang, T., Liu, N. S., Seet, L. F. & Hong, W. The emerging role of VHS domain-containing Tom1, Tom1L1 and Tom1L2 in membrane trafficking. Traffic 11, 1119–1128 (2010).
Liu, H. et al. ALKBH5-mediated m6A demethylation of GLUT4 mRNA promotes glycolysis and resistance to HER2-targeted therapy in breast cancer. Cancer Res. 82, 3974–3986 (2022).
Pulit, S. L. et al. Meta-analysis of genome-wide association studies for body fat distribution in 694 649 individuals of European ancestry. Hum. Mol. Genet. 28, 166–174 (2019).
Dunham, I. et al. An integrated encyclopedia of DNA elements in the human genome. Nature 489, 57–74 (2012).
Kundaje, A. et al. Integrative analysis of 111 reference human epigenomes. Nature 518, 317–330 (2015).
Ernst, J. & Kellis, M. ChromHMM: automating chromatin-state discovery and characterization. Nat. Methods 9, 215–216 (2012).
Kent, W. J. et al. The human genome browser at UCSC. Genome Res. 12, 996–1006 (2002).
Stumvoll, M., Van Haeften, T., Fritsche, A. & Gerich, J. Oral glucose tolerance test indexes for insulin sensitivity and secretion based on various availabilities of sampling times. Diabetes Care 24, 796–797 (2001).
Lindsay, T. et al. Descriptive epidemiology of physical activity energy expenditure in UK adults (The Fenland study). Int. J. Behav. Nutr. Phys. Act. 16, 126 (2019).
Hills, S. A. et al. The EGIR-RISC study (the European group for the study of insulin resistance: relationship between insulin sensitivity and cardiovascular disease risk): I. Methodology and objectives. Diabetologia 47, 566–570 (2004).
McCarthy, S. et al. A reference panel of 64,976 haplotypes for genotype imputation. Nat. Genet. 48, 1279–1283 (2016).
Auton, A. et al. A global reference for human genetic variation. Nature 526, 68–74 (2015).
Lim, E. T. et al. Distribution and medical impact of loss-of-function variants in the Finnish founder population. PLoS Genet. 10, e1004494 (2014).
FinnGen documentation of R3 release. https://finngen.gitbook.io/documentation/ (2020).
Winkler, T. W. et al. Quality control and conduct of genome-wide association meta-analyses. Nat. Protoc. 12, 1192–1212 (2014).
Willer, C. J., Li, Y. & Abecasis, G. R. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics 26, 2190–2191 (2010).
Yang, J. et al. Conditional and joint multiple-SNP analysis of GWAS summary statistics identifies additional variants influencing complex traits. Nat. Genet. 44, 369–375 (2012).
Explodecomputer/random-metal: adding random effects model to the METAL software. GitHub. https://github.com/explodecomputer/random-metal (2022).
Wakefield, J. Bayes factors for genome-wide association studies: comparison with P-values. Genet. Epidemiol. 33, 79–86 (2009).
Giambartolomei, C. et al. Bayesian test for colocalisation between pairs of genetic association studies using summary statistics. PLoS Genet. 10, e1004383 (2014).
Wang, Q. et al. Rare variant contribution to human disease in 281,104 UK Biobank exomes. Nature 597, 527–532 (2021).
Bulik-Sullivan, B. et al. LD score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat. Genet. 47, 291–295 (2015).
Finucane, H. K. et al. Heritability enrichment of specifically expressed genes identifies disease-relevant tissues and cell types. Nat. Genet. 50, 621 (2018).
Foley, C. N. et al. A fast and efficient colocalization algorithm for identifying shared genetic risk factors across multiple traits. Nat. Commun. 12, 764 (2021).
Zhu, C. H. et al. Cellular senescence in human myoblasts is overcome by human telomerase reverse transcriptase and cyclin-dependent kinase 4: consequences in aging muscle and therapeutic strategies for muscular dystrophies. Aging Cell 6, 515–523 (2007).
Fogarty, M. P., Cannon, M. E., Vadlamudi, S., Gaulton, K. J. & Mohlke, K. L. Identification of a regulatory variant that binds FOXA1 and FOXA2 at the CDC123/CAMK1D type 2 diabetes GWAS locus. PLoS Genet. 10, e1004633 (2014).
Roman, T. S. et al. A type 2 diabetes-associated functional regulatory variant in a pancreatic islet enhancer at the ADCY5 locus. Diabetes 66, 2521–2530 (2017).
Lotta, L. A. et al. Integrative genomic analysis implicates limited peripheral adipose storage capacity in the pathogenesis of human insulin resistance. Nat. Genet. 49, 17–26 (2017).
Leland Taylor, D. et al. Integrative analysis of gene expression, DNA methylation, physiological traits, and genetic variation in human skeletal muscle. Proc. Natl Acad. Sci. USA 116, 10883–10888 (2019).
Kuhn, R. M., Haussler, D. & Kent, W. J. The UCSC genome browser and associated tools. Brief. Bioinform. 14, 144–161 (2013).
Sun, W. et al. A transcriptomic analysis reveals novel patterns of gene expression during 3T3-L1 adipocyte differentiation. Front. Mol. Biosci. 7, 249 (2020).
Ng, Y., Ramm, G., Lopez, J. A. & James, D. E. Rapid activation of Akt2 is sufficient to stimulate GLUT4 translocation in 3T3-L1 adipocytes. Cell Metab. 7, 348–356 (2008).
Kohn, A. D., Summers, S. A., Birnbaum, M. J. & Roth, R. A. Expression of a constitutively active Akt Ser/Thr kinase in 3T3-L1 adipocytes stimulates glucose uptake and glucose transporter 4 translocation. J. Biol. Chem. 271, 31372–31378 (1996).
Tucker, D. F. et al. Isolation of state-dependent monoclonal antibodies against the 12-transmembrane domain glucose transporter 4 using virus-like particles. Proc. Natl Acad. Sci. USA 115, E4990–E4999 (2018).
Diaz-Vegas, A. et al. A high-content endogenous GLUT4 trafficking assay reveals new aspects of adipocyte biology. Life Sci. Alliance 6, e202201585 (2023).
Acknowledgements
We are grateful to investigators, staff members and study participants for their contribution to all participating studies. A full list of individual and study acknowledgments appears in the Supplementary Note. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Consortia
Contributions
A.W., X.Y., K.A.B., E.P.W., N.G., M.B., N.J.W., K.L.M., E.W., S.O’R and C.L. contributed to central analysis group. A.W., X.Y., K.A.B., A.U.J, M.W., N.J.W., K.L.M., E.W., S.O’R and C.L. contributed to follow-up analysis and interpretation. A.H.M., S.V. and K.L.M. contributed to SLC2A4 in vitro follow-up. A.W., D.M.N. and D.J.F. contributed to siRNA knockdown screen. A.W., X.Y., K.A.B., A.U.J, V.A., M.K.A., Z.A., L.L.B., S.R.B., M.P.B., T.A.B., Y-C.C., L-M.C., R-H.C., T.D.C., P.D., G.E.D., V.D.dM., J.D., O.P.D., M.R.E, L.F., T.M.F., C.G., M.O.G., X.G., S.G., L.H., U.H., G.H., S.H., K.H., K.Horn, W.A.H., Y-J.H., C-M.H., A.J., L.L.K., M.E.K., P.K., T.A.L., M.L., I-T.L., C.L., J.L., A.L., C-T.L., J’an.L., D.M., E.M., A.P. Moissl, A.P. Morris, N.N., N.P., A. Peters, R.B.P., R.N.R., K.R., C.R., C.S., K.S., M. Scholz, S. Sharma, S.E.S., S. Suleman, J. Tan, K.T., M.U., D.V., P.W., D.R.W., R.W., A.H.X., B.Z., E.A., M. Laakso, L.L., J.B.M., R.R., J.S., M.W., N.G. and N.J.W. contributed to study-level GWAS—analysis, phenotyping and genotyping. E.A., R.N.B., Y.C., F.S.C., T.F., J.C.F., A.F., H.G., L.G., T.H., H.A.K., P.K., M. Laakso, L.L., M. Loeffler, W.M., J.B.M., L.J.R., R.R., J.I.R., P.E.H.S., M. Stumvoll, J.S., A.T., T.T., J. Tuomilehto, R.W., M.W., N.G., M.B., N.J.W., K.L.M. and C.L. contributed to study-level oversight/PI. A.W., D.M.N., A.H.M., I.B., K.L.M., E.W., S.O’R, D.J.F. and C.L. contributed to writing group. All authors read, edited and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
I.B. and spouse declare stock ownership in GlaxoSmithKline, Incyte Ltd. and Inivata Ltd. J.C.F. has received consulting honoraria from Goldfinch Bio and AstraZeneca; speaker honoraria from Novo Nordisk, AstraZeneca and Merck for research lectures over which he had full control on content. M.E.K. is employed by SYNLAB Holding Deutschland GmbH. C.L. receives grants from Bayer Ag & Novo Nordisk and her husband works for Vertex. W.M. reports grants and personal fees from Siemens Diagnostics, Aegerion Pharmaceuticals, AMGEN, AstraZeneca, Danone Research, Sanofi, Pfizer, BASF and Numares; personal fees from Hoffmann LaRoche, MSD, Synageva; grants from Abbott Diagnostics, outside the submitted work. W.M. is employed by Synlab Holding Deutschland GmbH. J.B.M. serves as an Academic Associate for Quest Diagnostics. S.O’R. has undertaken remunerated consultancy work for Pfizer, AstraZeneca, GSK and ERX Pharmaceuticals. N.P. reports consulting honoraria from Bayer Vital GmbH and speaker honoraria from Novo Nordisk. J.S. is shareholder in Anagram kommunikation AB and Symptoms Europe AB, outside of the present study. D.V. has received research grants from Bayer A/S, Sanofi, Novo Nordisk A/S and Boehringer Ingelheim and holds shares in Novo Nordisk A/S. E.W. is now an employee of AstraZeneca. B.Z. is employed at the Swedish Medical Products Agency, SE-751 03 Uppsala, Sweden. The views expressed in this paper are the personal views of the authors and not necessarily the views of the Swedish government agency. All other authors declare no competing interests.
Peer review
Peer review information
Nature Genetics thanks Constantin Polychronakos, Miriam Udler and John Todd for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
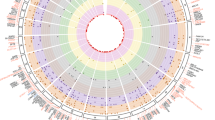
Extended Data Fig. 1 Overview of genetic discovery for insulin fold change and modified Stumvoll ISI, and downstream genetic and in vitro studies.
Created with BioRender.com.
Extended Data Fig. 2 Observational correlation of insulin fold change and modified Stumvoll ISI with metabolic traits in the Fenland study.
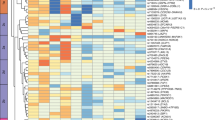
Pairwise Spearman’s rank correlation. Red shades denote positive correlation, blue shared denote negative correlation between trait pairs. X denotes no significant correlation (P > 0.05).
Extended Data Fig. 3 Observational correlation of insulin sensitivity and clearance related traits in the RISC study.
Pairwise Spearman’s rank correlation. Red shades denote positive correlation, blue shared denote negative correlation between trait pairs, legend along bottom of heatmap shows color scale relative to rho value. X denotes no significant correlation (P > 0.05). Abbreviations denote: IFC_OGTT – IFC calculated using OGTT measures, ISI_OGTT – Modified Stumvoll ISI calculated using OGTT measures. M_I – M/I index of insulin sensitivity measured by clamp. InsC0c – insulin measured at 0 min during clamp. InsO0c—insulin measured at 0 min during OGTT. InsC120c—insulin measured at 120 min during clamp. InsO120c—insulin measured at 120 min during OGTT. InsC_20c—insulin measured at 20 min before clamp. EGP_B—basal glucose production, EGP_SS—glucose production during clamp, GCR_B—basal glucose clearance, ml/min/kg lean body mass, GCR_SS—steady state glucose clearance, ml min−1 kg−1 lean body mass, icl_clamp—peripheral insulin clearance (1 min−1 m−2), icl_OGTT—endogenous ‘pre-hepatic’ clearance during the OGTT, hie_0—hepatic insulin extraction during clamp, OGIS—oral glucose insulin sensitivity index (ml min−1 m−2). ISR5dr—insulin secretion 5 mM glucose, beta cell dose response (pmol min−1 m−2). ISR0 basal insulin secretion (pmol min-1m-2). ISRtot—total insulin secretion (nmol m−2).
Extended Data Fig. 4 Meta-analysis workflow for genetic discovery analyses.
Analysis workflow for the meta-analysis of study-level GWAS results for Insulin fold change and modified Stumvoll ISI. Created with BioRender.com.
Extended Data Fig. 5 Two independent signals were identified at PPP1R3B for insulin fold change.
Conditional analyses identify a second independent signal at PPP1R3B for insulin fold change adjusted for BMI. The regional association plot shows the primary signal in red and the secondary signal in blue for marginal summary statistics for insulin fold change adjusted for BMI. Shade of point indicates pairwise linkage disequilibrium (R2) with indicated lead variant.
Extended Data Fig. 6 Forest plot of beta estimates for the association of rs60453193 with insulin fold change in individual cohorts.
Labels on the right-hand side indicate the ancestry of the study and study name. EUR- European ancestry, HIS-AMR—Hispanic American ancestry, EAS—East Asian ancestry. Left-hand side values are beta estimate and 95% confidence interval. Error bars denote a 95% confidence interval. X-axis denotes the beta estimate of associations with insulin fold change in BMI adjusted analyses.
Extended Data Fig. 7 Regional association plot at BICC1 (rs60453193) for insulin fold change in meta-analysis of non-European cohorts.
Unadjusted -log10 p-values are indicated on the y axis. Lead variant indicated by purple diamond.
Extended Data Fig. 8 rs117643180 exhibits allelic differences in transcription factor binding.
An EMSA using 6 µg per lane of nuclear extract from undifferentiated LHCN-M2 cells shows protein–DNA interactions for probes centered around each both alleles of rs117643180. The probe containing rs117643180-A shows allele-specific protein binding (arrow A, lane 6), relative to the probe containing rs117643180-C (lane 2). A 25-fold excess unlabeled probe containing the A allele competed away A-specific bands more effectively (lane 7) than 25-fold excess unlabeled probe containing the C allele (lane 8). Arrow B shows a biotinylated free probe (200 fmol per lane). Uncropped image is available in Source Data.
Extended Data Fig. 9 Confirmation of knockdown of positive control genes in wildtype 3T3-L1 adipocytes by western blot.
Representative blot from N = 2. Marker indicates protein size in kDa is outlined on the right-hand side of the blot. siGenome and OT+ represent siRNA pools with their corresponding targets indicated below (see Methods) and NT denotes non-targeting control. Antibodies are outlined on the left-hand side of the blot with Tubulin and B-actin used as loading controls. Uncropped blots are provided in Source Data.
Supplementary information
Supplementary Information
Supplementary Note—Study and Individual Acknowledgments, Supplementary Methods, Supplementary Results and Discussion and Supplementary Figs. 1–25.
Supplementary Tables
Supplementary Tables 1–33.
Source data
Source Data Extended Data Fig. 8 and Source Data Extended Data Fig. 9
Unprocessed ESMA blot corresponding to extended data figure 8. Unprocessed western blots corresponding to extended data figure 9
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Williamson, A., Norris, D.M., Yin, X. et al. Genome-wide association study and functional characterization identifies candidate genes for insulin-stimulated glucose uptake. Nat Genet 55, 973–983 (2023). https://doi.org/10.1038/s41588-023-01408-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41588-023-01408-9
This article is cited by
-
Causal relationship between diabetes mellitus, glycemic traits and Parkinson’s disease: a multivariable mendelian randomization analysis
Diabetology & Metabolic Syndrome (2024)
-
Dynamic measures of insulin action identify genetic determinants of dysglycemia
Nature Genetics (2023)