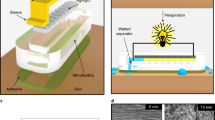
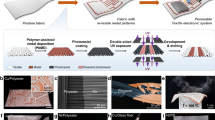
Abstract
Wearable electronics with great breathability enable a comfortable wearing experience and facilitate continuous biosignal monitoring over extended periods1,2,3. However, current research on permeable electronics is predominantly at the stage of electrode and substrate development, which is far behind practical applications with comprehensive integration with diverse electronic components (for example, circuitry, electronics, encapsulation)4,5,6,7,8. Achieving permeability and multifunctionality in a singular, integrated wearable electronic system remains a formidable challenge. Here we present a general strategy for integrated moisture-permeable wearable electronics based on three-dimensional liquid diode (3D LD) configurations. By constructing spatially heterogeneous wettability, the 3D LD unidirectionally self-pumps the sweat from the skin to the outlet at a maximum flow rate of 11.6 ml cm−2 min−1, 4,000 times greater than the physiological sweat rate during exercise, presenting exceptional skin-friendliness, user comfort and stable signal-reading behaviour even under sweating conditions. A detachable design incorporating a replaceable vapour/sweat-discharging substrate enables the reuse of soft circuitry/electronics, increasing its sustainability and cost-effectiveness. We demonstrated this fundamental technology in both advanced skin-integrated electronics and textile-integrated electronics, highlighting its potential for scalable, user-friendly wearable devices.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data supporting the results of this study are available in the paper and its Supplementary Information. Source data are provided with this paper.
Code availability
Custom codes for raw electrocardiogram signal collection are available at https://doi.org/10.5281/zenodo.10575185.
References
Huang, Q. & Zheng, Z. Pathway to developing permeable electronics. ACS Nano 16, 15537–15544 (2022).
Yan, Z. et al. Recent advances in breathable electronics. Nano Res. 16, 4130–4142 (2023).
Yang, J. et al. Toward a new generation of permeable skin electronics. Nanoscale 15, 3051–3078 (2023).
Miyamoto, A. et al. Inflammation-free, gas-permeable, lightweight, stretchable on-skin electronics with nanomeshes. Nat. Nanotechnol. 12, 907–913 (2017).
Tian, L. et al. Large-area MRI-compatible epidermal electronic interfaces for prosthetic control and cognitive monitoring. Nat. Biomed. Eng. 3, 194–205 (2019).
Ma, Z. et al. Permeable superelastic liquid-metal fibre mat enables biocompatible and monolithic stretchable electronics. Nat. Mater. 20, 859–868 (2021).
Jiang, Z. et al. A 1.3-micrometre-thick elastic conductor for seamless on-skin and implantable sensors. Nat. Electron. 5, 784–793 (2022).
Shi, X. et al. Large-area display textiles integrated with functional systems. Nature 591, 240–245 (2021).
Luo, Y. et al. Technology roadmap for flexible sensors. ACS Nano 17, 5211–5295 (2023).
Wang, C. et al. Continuous monitoring of deep-tissue haemodynamics with stretchable ultrasonic phased arrays. Nat. Biomed. Eng. 5, 749–758 (2021).
Chung, H. U. et al. Skin-interfaced biosensors for advanced wireless physiological monitoring in neonatal and pediatric intensive-care units. Nat. Med. 26, 418–429 (2020).
Guan, Y.-S. et al. Elastic electronics based on micromesh-structured rubbery semiconductor films. Nat. Electron. 5, 881–892 (2022).
Jiang, Y. et al. A universal interface for plug-and-play assembly of stretchable devices. Nature 614, 456–462 (2023).
Li, J. et al. Ultrathin, soft, radiative cooling interfaces for advanced thermal management in skin electronics. Sci. Adv. 9, eadg1837 (2023).
Song, E. et al. Miniaturized electromechanical devices for the characterization of the biomechanics of deep tissue. Nat. Biomed. Eng. 5, 759–771 (2021).
Cheng, X. et al. Programming 3D curved mesosurfaces using microlattice designs. Science 379, 1225–1232 (2023).
Jung, D. et al. Highly conductive and elastic nanomembrane for skin electronics. Science 373, 1022–1026 (2021).
Shimura, T., Sato, S., Zalar, P. & Matsuhisa, N. Engineering the comfort-of-wear for next generation wearables. Adv. Electron. Mater. 9, 2200512 (2023).
Chen, F., Huang, Q. & Zheng, Z. Permeable conductors for wearable and on-skin electronics. Small Struct. 3, 2100135 (2022).
Li, Q. et al. Highly thermal-wet comfortable and conformal silk-based electrodes for on-skin sensors with sweat tolerance. ACS Nano 15, 9955–9966 (2021).
Cheng, S. et al. Ultrathin hydrogel films toward breathable skin-integrated electronics. Adv. Mater. 35, 2206793 (2023).
Kim, Y. et al. Chip-less wireless electronic skins by remote epitaxial freestanding compound semiconductors. Science 377, 859–864 (2022).
Peng, X. et al. A breathable, biodegradable, antibacterial, and self-powered electronic skin based on all-nanofiber triboelectric nanogenerators. Sci. Adv. 6, eaba9624 (2020).
Lee, S. et al. Nanomesh pressure sensor for monitoring finger manipulation without sensory interference. Science 370, 966–970 (2020).
Yan, W. et al. Single fibre enables acoustic fabrics via nanometre-scale vibrations. Nature 603, 616–623 (2022).
Yeon, H. et al. Long-term reliable physical health monitoring by sweat pore-inspired perforated electronic skins. Sci. Adv. 7, eabg8459 (2021).
Wang, Y. et al. Robust, self-adhesive, reinforced polymeric nanofilms enabling gas-permeable dry electrodes for long-term application. Proc. Natl Acad. Sci. 118, e2111904118 (2021).
Nayeem, M. O. G. et al. All-nanofiber-based, ultrasensitive, gas-permeable mechanoacoustic sensors for continuous long-term heart monitoring. Proc. Natl Acad. Sci. 117, 7063–7070 (2020).
Yan, Z. et al. Highly stretchable van der Waals thin films for adaptable and breathable electronic membranes. Science 375, 852–859 (2022).
Jang, K.-I. et al. Rugged and breathable forms of stretchable electronics with adherent composite substrates for transcutaneous monitoring. Nat. Commun. 5, 4779 (2014).
Xiong, J. et al. Skin-touch-actuated textile-based triboelectric nanogenerator with black phosphorus for durable biomechanical energy harvesting. Nat. Commun. 9, 4280 (2018).
Chen, J. et al. Micro-cable structured textile for simultaneously harvesting solar and mechanical energy. Nat. Energy 1, 16138 (2016).
Zhu, Y. et al. A breathable, passive-cooling, non-inflammatory, and biodegradable aerogel electronic skin for wearable physical-electrophysiological-chemical analysis. Adv. Mater. 35, 2209300 (2023).
Zheng, S. et al. Moisture-wicking, breathable, and intrinsically antibacterial electronic skin based on dual-gradient poly(ionic liquid) nanofiber membranes. Adv. Mater. 34, 2106570 (2022).
Wang, Y. et al. A durable nanomesh on-skin strain gauge for natural skin motion monitoring with minimum mechanical constraints. Sci. Adv. 6, eabb7043 (2020).
Kim, K. K. et al. A substrate-less nanomesh receptor with meta-learning for rapid hand task recognition. Nat. Electron. 6, 64–75 (2023).
Chen, H. et al. Continuous directional water transport on the peristome surface of Nepenthes alata. Nature 532, 85–89 (2016).
Li, J. et al. Topological liquid diode. Sci. Adv. 3, eaao3530 (2017).
Taylor, N. A. S. & Machado-Moreira, C. A. Regional variations in transepidermal water loss, eccrine sweat gland density, sweat secretion rates and electrolyte composition in resting and exercising humans. Extrem. Physiol. Med. 2, 4 (2013).
Smith, C. J. & Havenith, G. Body mapping of sweating patterns in male athletes in mild exercise-induced hyperthermia. Eur. J. Appl. Physiol. 111, 1391–1404 (2011).
Godek, S. F., Bartolozzi, A. R. & Godek, J. J. Sweat rate and fluid turnover in American football players compared with runners in a hot and humid environment. Br. J. Sports Med. 39, 205–211 (2005).
Chung, H. U. et al. Binodal, wireless epidermal electronic systems with in-sensor analytics for neonatal intensive care. Science 363, eaau0780 (2019).
Lao, L., Shou, D., Wu, Y. S. & Fan, J. T. “Skin-like” fabric for personal moisture management. Sci. Adv. 6, eaaz0013 (2020).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant no. 62122002), City University of Hong Kong (grant nos. 9667221, 9678274 and 9610444), as part of the InnoHK Project 2.2–AI-based 3D ultrasound imaging algorithm at Hong Kong Centre for Cerebro-cardiovascular Health Engineering (COCHE), the Research Grants Council of the Hong Kong Special Administrative Region (grants nos. 11213721, 11215722, 11211523 and RFS2324-1S03), Shenzhen Science and Technology Innovation Commission (grant no. SGDX20220530111401011) and the RGC Senior Research Fellow Scheme (SRFS2122-5S04).
Author information
Authors and Affiliations
Contributions
X.Y. and B.Z. conceived the idea and designed the projects. B.Z. designed the whole system and conducted overall experiments. B.Z. and Jiy.L. designed the liquid diode. J.Z. designed the circuits. L.C. and B.Z. conducted the mechanical modelling. Q.Z. and B.Z. carried out the cytotoxicity test. Jiy.L., Z.M. and B.Z. conducted the fluid simulation. F.C., L.C. and X.H. performed the materials characterization. Y.Y., C.K.Y., Jia.L., P.W., S.J., H.L., D.L., Y.L., K.Y., R.S. and Z.C. assisted in the fabrication and testing experiments. B.Z., L.C., Y.H., G.Z., J.Z., Jiy.L. and Y.G. organized the user study. B.L.K., W.Y., F.W., Z.Z. and Z.W. evaluated the experiments and contributed valuable ideas. B.Z. and X.Y. wrote the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Nanshu Lu, Sheng Xu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Contact angle of the selective plasma-treated fabric under various treatment conditions.
a, Schematic illustration of the fabric samples for selective plasma treatment. b, Contact angle of the channels with mask size from 0.2 to 1.5 mm on both top (T) and back (B) sides of the superhydrophobic fabric after plasma treatment for 0.5 to 10 min.
Extended Data Fig. 2 Fluid simulation of the 3D LD.
a, Velocity magnitude. b, Streamlines. c, Pressure contours. d, Spatiotemporal variation in the liquid volume fraction.
Extended Data Fig. 3 Cytotoxicity test of the VLD and 3D LD.
a, LIVE/DEAD staining (green, live cell; red, dead cell). Scale bars, 100 μm. b,c, Cell viability (b) and optical density (OD) values (c) obtained in the in vitro cytotoxicity assay. Bar height, mean; error bars, s.d.; n = 3 independent samples.
Extended Data Fig. 4 Evaluation of skin temperature beneath various patches.
a, Sequential arrangement of patches from left to right: commercial ECG electrodes (1), PDMS (2), VLD (3) and 3D LD (4). Scale bar, 5 cm. b, Outline of the test procedure. c,d, Photographic and infrared images of skin pre-exercise and post-exercise (PE) with different patches applied. Scale bars, 5 cm.
Extended Data Fig. 5 Optical images and FEA modelling of the permeable ECG monitor under stretching, bending and twisting.
a, Optical images. b, FEA modelling. Scale bars, 1 cm.
Extended Data Fig. 6 Comparison of motion artefacts between the conventional cable-connected type and our wearable device.
a–d, Raw ECG data (a,b) and post-processed data (c,d) from the commercial electrode connected with the DAQ and our device under resting, walking and jumping conditions. ECG data from the DAQ were lost under walking and jumping conditions, whereas our device maintained stable ECG signal readout under body motion.
Extended Data Fig. 7 Continuous ECG recording during a 30-min cycling workout.
a, ECG signals from the cable-connected DAQ are difficult to discern during the cycling process. b, Continuous ECG signals from our wearable and permeable device. The HR and QTO are calculated simultaneously and continuously. c, Enlarged view of the ECG signal, HR and QTO during the cycling and rest periods.
Extended Data Fig. 8 Comparative assessment of the thermal comfort provided by commercial ECG electrodes and our permeable ECG monitor.
Three volunteers were asked to wear both the commercial electrodes and our device while continuously cycling for 30 min. Infrared images of the skin are captured immediately after patch removal and following a 5-min rest. The maximum temperature beneath the commercial electrodes was nearly 1.9 °C higher than that beneath our device. Scale bars, 5 cm.
Extended Data Fig. 9 ECG monitoring by the permeable patch before and after running.
a,b, Optical images of the patches attached to the chest before (a) and after (b) exercise. Scale bars, 5 cm. c, Optical image depicting the condition of skin beneath the device. Scale bar, 1 cm. d, Optical image demonstrating the sweat-discharging channels of the device. Scale bar, 1 cm. e,f, ECG signals acquired before (e) and after exercise (f).
Extended Data Fig. 10 Four-layer integrated circuit.
a, Exploded schematic illustrating the layer-by-layer structure. b, Photographic image of the integrated circuit. Scale bar, 1 cm. c,d, Images of the permeable device affixed to the chest (c) and forearm (d) for ECG (c) and EMG (d) signal acquisition, alongside the recorded ECG (c) and EMG (d) traces. Scale bars, 6 cm.
Supplementary information
Supplementary Video 1
Anti-gravity sweat transport of the VLD.
Supplementary Video 2
Directional sweat transport of the HLD.
Supplementary Video 3
Fluid simulation of the 3D LD.
Supplementary Video 4
Gas and sweat permeability of the PDMS and 3D LD.
Supplementary Video 5
Deformation of the wearable sweat-permeable ECG monitor.
Supplementary Video 6
Demonstration of the sweat-permeable ECG monitor.
Supplementary Video 7
Continuous ECG monitoring during daily activities and exercise.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, B., Li, J., Zhou, J. et al. A three-dimensional liquid diode for soft, integrated permeable electronics. Nature 628, 84–92 (2024). https://doi.org/10.1038/s41586-024-07161-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-024-07161-1
This article is cited by
-
The wearable electronic patch that’s impervious to sweat
Nature (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.