Abstract
Plasma cells produce large quantities of antibodies and so play essential roles in immune protection1. Plasma cells, including a long-lived subset, reside in the bone marrow where they depend on poorly defined microenvironment-linked survival signals1. We show that bone marrow plasma cells use the ligand-gated purinergic ion channel P2RX4 to sense extracellular ATP released by bone marrow osteoblasts through the gap-junction protein pannexin 3 (PANX3). Mutation of Panx3 or P2rx4 each caused decreased serum antibodies and selective loss of bone marrow plasma cells. Compared to their wild-type counterparts, PANX3-null osteoblasts secreted less extracellular ATP and failed to support plasma cells in vitro. The P2RX4-specific inhibitor 5-BDBD abrogated the impact of extracellular ATP on bone marrow plasma cells in vitro, depleted bone marrow plasma cells in vivo and reduced pre-induced antigen-specific serum antibody titre with little posttreatment rebound. P2RX4 blockade also reduced autoantibody titre and kidney disease in two mouse models of humoral autoimmunity. P2RX4 promotes plasma cell survival by regulating endoplasmic reticulum homeostasis, as short-term P2RX4 blockade caused accumulation of endoplasmic reticulum stress-associated regulatory proteins including ATF4 and B-lineage mutation of the pro-apoptotic ATF4 target Chop prevented bone marrow plasma cell demise on P2RX4 inhibition. Thus, generating mature protective and pathogenic plasma cells requires P2RX4 signalling controlled by PANX3-regulated extracellular ATP release from bone marrow niche cells.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Full scans for gels are provided in Supplementary Fig. 1. Source data are provided with this paper.
References
Radbruch, A. et al. Competence and competition: the challenge of becoming a long-lived plasma cell. Nat. Rev. Immunol. 6, 741–750 (2006).
Jarvis, M. F. & Khakh, B. S. ATP-gated P2X cation-channels. Neuropharmacology 56, 208–215 (2009).
Borges da Silva, H. et al. The purinergic receptor P2RX7 directs metabolic fitness of long-lived memory CD8(+) T cells. Nature 559, 264–268 (2018).
Burnstock, G. P2X ion channel receptors and inflammation. Purinergic Signal. 12, 59–67 (2016).
Ishikawa, M. et al. Pannexin 3 functions as an ER Ca(2+) channel, hemichannel and gap junction to promote osteoblast differentiation. J. Cell Biol. 193, 1257–1274 (2011).
Ishikawa, M. et al. Pannexin 3 and connexin 43 modulate skeletal development through their distinct functions and expression patterns. J. Cell Sci. 129, 1018–1030 (2016).
Wilmore, J. R., Jones, D. D. & Allman, D. Protocol for improved resolution of plasma cell subpopulations by flow cytometry. Eur. J. Immunol. 47, 1386–1388 (2017).
Jones, D. D. et al. mTOR has distinct functions in generating versus sustaining humoral immunity. J. Clin. Invest. 126, 4250–4261 (2016).
Cassese, G. et al. Plasma cell survival is mediated by synergistic effects of cytokines and adhesion-dependent signals. J. Immunol. 171, 1684–1690 (2003).
Burnstock, G. Purine and pyrimidine receptors. Cell. Mol. Life Sci. 64, 1471–1483 (2007).
Hohenegger, M. et al. Gsalpha-selective G protein antagonists. Proc. Natl Acad. Sci. USA 95, 346–351 (1998).
Jones, C. A. et al. Functional characterization of the P2X(4) receptor orthologues. Br. J. Pharmacol. 129, 388–394 (2000).
Heng, T. S., Painter, M. W. & The Immunological Genome Project Consortium. The Immunological Genome Project: networks of gene expression in immune cells. Nat. Immunol. 9, 1091–1094 (2008).
Tabula Sapiens Consortium. The tabula sapiens: a multiple-organ, single-cell transcriptomic atlas of humans. Science 376, eabl4896 (2022).
Hobeika, E. et al. Testing gene function early in the B cell lineage in mb1-cre mice. Proc. Natl Acad. Sci. USA 103, 13789–13794 (2006).
Khalil, A. M., Cambier, J. C. & Shlomchik, M. J. B cell receptor signal transduction in the GC is short-circuited by high phosphatase activity. Science 336, 1178–1181 (2012).
Ahuja, A., Anderson, S. M., Khalil, A. & Shlomchik, M. J. Maintenance of the plasma cell pool is independent of memory B cells. Proc. Natl Acad. Sci. USA 105, 4802–4807 (2008).
Yannoutsos, N. et al. A cis element in the recombination activating gene locus regulates gene expression by counteracting a distant silencer. Nat. Immunol. 5, 443–450 (2004).
Hoyer, B. F. et al. Short-lived plasmablasts and long-lived plasma cells contribute to chronic humoral autoimmunity in NZB/W mice. J. Exp. Med. 199, 1577–1584 (2004).
Morris, S. C., Cheek, R. L., Cohen, P. L. & Eisenberg, R. A. Autoantibodies in chronic graft versus host result from cognate T–B interactions. J. Exp. Med. 171, 503–517 (1990).
Koshimizu, T. A. et al. Characterization of calcium signaling by purinergic receptor-channels expressed in excitable cells. Mol. Pharmacol. 58, 936–945 (2000).
Bettigole, S. E. & Glimcher, L. H. Endoplasmic reticulum stress in immunity. Annu. Rev. Immunol. 33, 107–138 (2015).
Han, J. et al. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat. Cell Biol. 15, 481–490 (2013).
Puthalakath, H. et al. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell 129, 1337–1349 (2007).
Wilmore, J. R. & Allman, D. Here, there and anywhere? Arguments for and against the physical plasma cell survival niche. J. Immunol. 199, 839–845 (2017).
Robinson, M. J. et al. Intrinsically determined turnover underlies broad heterogeneity in plasma-cell lifespan. Immunity 56, 1596–1612 (2023).
Benson, M. J. et al. Cutting edge: the dependence of plasma cells and independence of memory B cells on BAFF and APRIL. J. Immunol. 180, 3655–3659 (2008).
O’Connor, B. P. et al. BCMA is essential for the survival of long-lived bone marrow plasma cells. J. Exp. Med. 199, 91–98 (2004).
Peperzak, V. et al. Mcl-1 is essential for the survival of plasma cells. Nat. Immunol. 14, 290–297 (2013).
Nikbakht, N., Migone, T. S., Ward, C. P. & Manser, T. Cellular competition independent of BAFF/B lymphocyte stimulator results in low frequency of an autoreactive clonotype in mature polyclonal B cell compartments. J. Immunol. 187, 37–46 (2011).
Vincent, F. B., Saulep-Easton, D., Figgett, W. A., Fairfax, K. A. & Mackay, F. The BAFF/APRIL system: emerging functions beyond B cell biology and autoimmunity. Cytokine Growth Factor Rev. 24, 203–215 (2013).
Mariathasan, S. et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440, 228–232 (2006).
Suurvali, J., Boudinot, P., Kanellopoulos, J. & Ruutel Boudinot, S. P2X4: a fast and sensitive purinergic receptor. Biomed. J. 40, 245–256 (2017).
Fujisaki, J. et al. In vivo imaging of Treg cells providing immune privilege to the haematopoietic stem-cell niche. Nature 474, 216–219 (2011).
Glatman Zaretsky, A. et al. T regulatory cells support plasma cell populations in the bone marrow. Cell Rep. 18, 1906–1916 (2017).
Zinszner, H. et al. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 12, 982–995 (1998).
Tellier, J. et al. Blimp-1 controls plasma cell function through the regulation of immunoglobulin secretion and the unfolded protein response. Nat. Immunol. 17, 323–330 (2016).
Slifka, M. K., Antia, R., Whitmire, J. K. & Ahmed, R. Humoral immunity due to long-lived plasma cells. Immunity 8, 363–372 (1998).
Raje, N. et al. Anti-BCMA CAR T-cell therapy bb2121 in relapsed or refractory multiple myeloma. N. Engl. J. Med. 380, 1726–1737 (2019).
Seckinger, A. et al. Target expression, generation, preclinical activity and pharmacokinetics of the BCMA-T cell bispecific antibody EM801 for multiple myeloma treatment. Cancer Cell 31, 396–410 (2017).
Neubert, K. et al. The proteasome inhibitor bortezomib depletes plasma cells and protects mice with lupus-like disease from nephritis. Nat. Med. 14, 748–755 (2008).
Obeng, E. A. et al. Proteasome inhibitors induce a terminal unfolded protein response in multiple myeloma cells. Blood 107, 4907–4916 (2006).
Woodle, E. S., Tremblay, S. & Driscoll, J. Targeting plasma cells with proteasome inhibitors: principles from primates. J. Am. Soc. Nephrol. 28, 1951–1953 (2017).
Cheng, Q. et al. Selective depletion of plasma cells in vivo based on the specificity of their secreted antibodies. Eur. J. Immunol. 50, 284–291 (2020).
Ralevic, V. & Burnstock, G. Receptors for purines and pyrimidines. Pharmacol. Rev. 50, 413–492 (1998).
Iwamoto, T. et al. Pannexin 3 regulates intracellular ATP/cAMP levels and promotes chondrocyte differentiation. J. Biol. Chem. 285, 18948–18958 (2010).
Wolf, F. A., Angerer, P. & Theis, F. J. SCANPY: large-scale single-cell gene expression data analysis. Genome Biol. 19, 15 (2018).
Stuart, T. et al. Comprehensive integration of single-cell data. Cell 177, 1888–1902 (2019).
Lopez, R., Regier, J., Cole, M. B., Jordan, M. I. & Yosef, N. Deep generative modeling for single-cell transcriptomics. Nat. Methods 15, 1053–1058 (2018).
Traag, V. A., Waltman, L. & van Eck, N. J. From Louvain to Leiden: guaranteeing well-connected communities. Sci. Rep. 9, 5233 (2019).
Acknowledgements
We thank M. Cancro, A. Nussenzweig, S. John and V. Smith-Shapiro for reviewing this manuscript, D. Hildeman and A. Marshak-Rothstein for helpful discussions and M. Shlomchik for providing CD20-TAM-Cre mice. We also thank M. Cam and D. Fonseca for help with statistics, H. Averman for help with histological analysis and the UPenn Flow Cytometry and Cell Sorting facility. This work was supported by NIH grant nos. R21 AI161931, RO1 AI139123 and RO1 AI175185 and an ASPIRE award from the Mark Foundation for Cancer Research to D.A., KAKENHI of Japan grant nos. 16KK0196 and 19K22698 to M.I., NIH grant nos. T32-HL07439 and T32AI007632 to Z.H. and J.L., respectively, an Amyloidosis Foundation Research Grant to Z.H. and the Intramural Research Program of the Center for Cancer Research at the NCI (A.B.).
Author information
Authors and Affiliations
Contributions
M.I., D.A. and A.B. designed the experiments. M.I., Z.H., Y.Z., A.D., C.R. and J.L. performed the experiments including all ELISPOT, ELISA and flow cytometry studies. M.L. performed bioinformatic analyses. M.I., D.A. and A.B. wrote the manuscript with input from all other co-authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Shiv Pillai and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
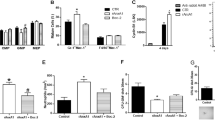
Extended Data Fig. 1 Panx3 mutation does not perturb developing or mature myeloid and lymphoid cell populations.
(a) Numbers of viable BM cells in 16-week-old Panx3+/+ and Panx3−/− mice. (b–e) Evaluation of the indicated BM myeloid and B-lineage cells (b), thymocytes (c), T and B cells in spleen (d) and peritoneal B1 cells (e). (b) Numbers of macrophages (CD115− Gr1− F4/80+), Gr1+ and Gr1− monocytes (CD115+ Gr1high, CD115− Gr1low F4/80+/− respectively), neutrophils (CD115− Gr1high), pro- & pre-B cells (CD19+ IgM− CD43+/−) and immature (CD19+ IgM+ IgD−) and mature (CD19+ IgM+ IgD+) B cells. (c-e) Numbers of CD4 and CD8 single positive thymocytes (c), splenic CD4 and CD8 T cells and mature (CD19+ IgD+) B cells (d) and peritoneal cavity CD19 + CD43 + B1 B cells (e) in 5-month-old Panx3+/+ and Panx3−/− mice. (See Extended Figs. 2 and 3 for flow cytometry gating strategies for BM and spleen cells). (n = 6/grp for panels a-d, n = 3/grp for panel e). (f, g) CD138+ Sca-1+ PCs in the BM (f) and spleen (g) in 16-week-old Panx3+/+ and Panx3−/− mice (n = 6/grp). Plots in (f) show additional analysis of newly formed (B220+) and mature (B220−) PCs. All bar graphs are means. For panels (a,b), *, p = 0.0022. All p values were derived from two-tailed Mann–Whitney tests, without any adjustment for multiple comparisons. The experiments in panels (a-d) and (f-g) were performed at least thrice and the experiments in panel (e) were performed twice.
Extended Data Fig. 4 Panx3 and eATP support BM PCs.
(a) Schematic for experiments wherein fresh BM cells were cultured with pre-induced osteoblastic cells for 4 days before ELISPOT analyses. (b) Quantitative RT-PCR for CXCL12, SCF, IL6 and APRIL expression with osteoblastic calvarial cells from Panx3+/+ and Panx3−/− mice after 14 days culture as in (a). Graph shows means for technical replicates; n = 3. A control Panx3+/+ sample was set to 1 for each set of measurements and the other measurements were recalculated relative to that sample. All p values were derived from two-tailed Mann–Whitney tests, without any adjustment for multiple comparisons. The experiments in panel b were performed twice.
Extended Data Fig. 5 P2rX inhibitors deplete BM PCs.
(a) Representative flow cytometry data for BM PCs in WT mice given DMSO alone (Control) or Suramin (2.0 mg/kg), PPADS (2.5 mg/kg), or both Suramin and PPADS thrice over seven days before analysis. Representative of 3 mice per group and three separate experiments. (b) Expression of P2rX and P2rYfamily members in BM PCs (ImmGen data, see Methods). (c) Annotated UMAP visualizing naïve and memory B cells and plasma cells identified in adult human bone marrow (left) with Heatmap visualizing averaged and gene-wise scaled gene expression for each population (right) (see Methods).
Extended Data Fig. 6 Selective impact of B-lineage P2rX4 mutation on BM PCs.
(a) Flow cytometric analyses for BM B220+/− CD138high PCs and numbers of splenic PCs (Dump- IgD- CD138+ Sca-1+) (b, c) Cell numbers for plasma cells in spleen (b) or indicated cell populations in spleen and BM (c) and peritoneal cavity CD19+ CD43+ B1 B cells (d) in 5-month-old WT;Mb1-Cre and P2rX4f/f;Mb1-Cre adults. (For panels (a-c) n = 6/group and for panel (d) n = 3/group). (e,f) Analysis of B220+/− CD138high BM PCs (e) and all splenic PCs (CD138high Sca-1+) (f) in WT;CD20-TAM-Cre and P2rX4f/f; CD20-TAM-Cre adults. (g) Numbers of the indicated T and B cell subsets in the spleen and developing B cells in BM of the indicated mice. (h) ELISPOT analyses of IgG-secreting BM cells from CD20-TAM-Cre and P2rX4f/f;CD20-TAM-Cre mice fed tamoxifen-laced chow for 4 weeks previously. BM cells from tamoxifen-fed mice were cultured for 2 days with or without adding 100μM ATP before addition to ELISPOT assay. For panels (e-h) n = 6/grp. All bar graphs are means. For panels (a-h) experiments were performed at least thrice. *, p = 0.0022. All p values were derived from two-tailed Mann–Whitney tests, without any adjustment for multiple comparisons.
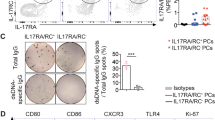
Extended Data Fig. 7 Induced B-lineage-restricted P2rX4 mutation in mature BM PCs.
(a) Cre expression in B220− BM LLPCs of CD20-TAM-Cre adults. Data are representative plots of n = 3 mice/grp. (b) P2rX4 genomic PCR for sorted T cells, B cells and B220− PCs of CD20-TAM-Cre adult mice fed tamoxifen for 1 week (n = 3). HIF1α used as a DNA loading control. BM B220− PCs were sorted sequentially twice to ensure purity. For gel source data, see Supplementary Fig. 1. (c) NP-specific IgG+ PCs in the spleen of the indicated mice. All mice were given tamoxifen-laced chow for 4 weeks beginning at 5 weeks of age, then immunized with NP-KLH/alum and analysed by ELISPOT 30 days later. Error bars represent the mean (n = 5/grp). (d) NP-specific GC B cells in separate tamoxifen-fed WT;CD20-TAM-Cre and P2rX4f/f; CD20-TAM-Cre adults immunized 14 days previously. Graph shows means for hapten-binding GC B cells for 3 mice/group. For a comprehensive illustration of parent gates for evaluating NP-specific GC B cells see Extended Data Fig. 3. Experiments in every panel including the PCR data in (b) were performed twice. All p values were derived from two-tailed Mann–Whitney tests for all plots, without any adjustment for multiple comparisons.
Extended Data Fig. 8 Differential impact of P2rX4 inhibition on BM PCs versus naïve and GC B cells.
Flow cytometric analysis of BM and spleen cells from B6 adult females given 5-BDBD (4.25 mg/kg) or DMSO alone as control every 2 days i.v. 4 times. All analyses occurred 8 days after the first dose. (a-c) Shown are steady-state B220+/− BM PCs (a), splenic PCs (b) and naïve splenic T and B cells and BM B-lineage cells (c) from unimmunized mice. For panels (a-c) n = 6/grp from one of three experiments. (d) NP-specific GC B cells in separate mice immunized 14 days before with NP-KLH (n = 3/grp, representative of two separate experiments). For a comprehensive illustration of parent gates for evaluating NP-specific GC B cells see Extended Data Fig. 3. (e) Numbers and representative flow cytometric analysis of peritoneal cavity CD19+ IgM+ CD43+ B1 B cells in control and 5-BDBD treated C57BL/6 adults. n = 4/group. All bar graphs are means. All p values were derived from two-tailed Mann–Whitney tests for all plots, without any adjustment for multiple comparisons.
Extended Data Fig. 9 Rapid recovery of serum antibody titre and proteinuria following rapamycin.
NZB/W mice were monitored for serum dsDNA-specific IgG titre (a) and urine protein levels (b) over the indicated time frame. Protein scores were graded on a semiquantitative scale: 1, ≥30 mg/dl protein; 2, ≥100 mg/dl; 3, ≥300 mg/dl; and 4, ≥2,000 mg/dl. Twice weekly rapamycin (20 mg/kg) was administered i.v. from 29 to 33 weeks old age. Data represent means and are representative of 2 separate experiments each using 4 mice per group. Treatment windows are shown with grey rectangles.
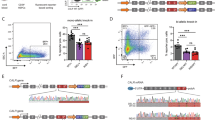
Extended Data Fig. 10 Induced CHOP mutation in mature BM PCs.
Genomic DNA was prepared from sorted cells collected from CHOPf/f;CD20-TAM-Cre adults that were fed tamoxifen-laced chow for 4 weeks and evaluated the following day (n = 3). Sorted cell populations were mature BM PCs (CD138high Sca-1+ B220−) and B (surface IgM+) and T (CD3+) cells that were then subjected to PCR to amplify the CHOP (top) or HIF1α locus. For gel source data, see Supplementary Fig. 1.
Supplementary information
Supplementary Fig. 1
Uncropped gels for genomic PCR analyses.
Supplementary Table 1
List of oligonucleotides used for genomic PCR and qPCR analyses.
Source data
Rights and permissions
About this article
Cite this article
Ishikawa, M., Hasanali, Z.S., Zhao, Y. et al. Bone marrow plasma cells require P2RX4 to sense extracellular ATP. Nature 626, 1102–1107 (2024). https://doi.org/10.1038/s41586-024-07047-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-024-07047-2
This article is cited by
-
Osteoblast-derived ATP maintains bone marrow plasma cells
Nature Reviews Immunology (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.