Abstract
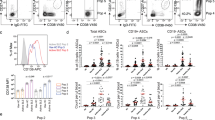
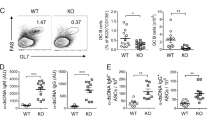
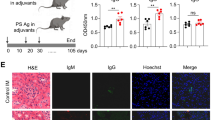
Recent studies have demonstrated a central role for plasma cells in the development of autoimmune diseases, such as systemic lupus erythematosus (SLE). Currently, both the phenotypic features and functional regulation of autoreactive plasma cells during SLE pathogenesis remain largely unclear. In this study, we first found that a major subset of IL-17 receptor-expressing plasma cells potently produced anti-dsDNA IgG upon IL-17A (IL-17) stimulation in SLE patients and lupus mice. Using a humanized lupus mouse model, we showed that the transfer of Th17 cell-depleted PBMCs from lupus patients resulted in a significantly reduced plasma cell response and attenuated renal damage in recipient mice compared to the transfer of total SLE PBMCs. Moreover, long-term BrdU incorporation in lupus mice detected highly enriched long-lived BrdU+ subsets among IL-17 receptor-expressing plasma cells. Lupus mice deficient in IL-17 or IL-17 receptor C (IL-17RC) exhibited a diminished plasma cell response and reduced autoantibody production with attenuated renal damage, while the adoptive transfer of Th17 cells triggered the plasma cell response and renal damage in IL-17-deficient lupus mice. In reconstituted chimeric mice, IL-17RC deficiency resulted in severely impaired plasma cell generation but showed no obvious effect on germinal center B cells. Further mechanistic studies revealed that IL-17 significantly promoted plasma cell survival via p38-mediated Bcl-xL transcript stabilization. Together, our findings identified a novel function of IL-17 in enhancing plasma cell survival for autoantibody production in lupus pathogenesis, which may provide new therapeutic strategies for the treatment of SLE.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Tsokos, G. C. Systemic lupus erythematosus. N. Engl. J. Med. 365, 2110–2121 (2011).
Malkiel, S., Barlev, A. N., Atisha-Fregoso, Y., Suurmond, J. & Diamond, B. Plasma cell differentiation pathways in systemic lupus erythematosus. Front. Immunol. 9, 427 (2018).
Hiepe, F. et al. Long-lived autoreactive plasma cells drive persistent autoimmune inflammation. Nat. Rev. Rheumatol. 7, 170–178 (2011).
Cheng, Q. et al. Autoantibodies from long-lived ‘memory’ plasma cells of NZB/W mice drive immune complex nephritis. Ann. Rheum. Dis. 72, 2011–2017 (2013).
Mahevas, M., Michel, M., Weill, J. C. & Reynaud, C. A. Long-lived plasma cells in autoimmunity: lessons from B-cell depleting therapy. Front. Immunol. 4, 494 (2013).
Ma, K. et al. The expanding functional diversity of plasma cells in immunity and inflammation. Cell Mol. Immunol. 17, 421–422 (2020).
Ma, K. et al. TLR4(+)CXCR4(+) plasma cells drive nephritis development in systemic lupus erythematosus. Ann. Rheum. Dis. 77, 1498–1506 (2018).
Pioli, P. D., Casero, D., Montecino-Rodriguez, E., Morrison, S. L. & Dorshkind, K. Plasma cells are obligate effectors of enhanced myelopoiesis in aging bone marrow. Immunity 51, 351–366 e356 (2019).
Shi, W. et al. Transcriptional profiling of mouse B cell terminal differentiation defines a signature for antibody-secreting plasma cells. Nat. Immunol. 16, 663–673 (2015).
Beringer, A. & Miossec, P. Systemic effects of IL-17 in inflammatory arthritis. Nat. Rev. Rheumatol. 15, 491–501 (2019).
Hsu, H. C. et al. Interleukin 17-producing T helper cells and interleukin 17 orchestrate autoreactive germinal center development in autoimmune BXD2 mice. Nat. Immunol. 9, 166–175 (2008).
Pisitkun, P. et al. Interleukin-17 cytokines are critical in development of fatal lupus glomerulonephritis. Immunity 37, 1104–1115 (2012).
Shah, K. et al. Dysregulated balance of Th17 and Th1 cells in systemic lupus erythematosus. Arthritis Res. Ther. 12, R53 (2010).
Salem, D., Subang, R., Kuwana, M., Levine, J. S. & Rauch, J. T cells from induced and spontaneous models of SLE recognize a common T cell epitope on beta2-glycoprotein I. Cell Mol. Immunol. 16, 685–693 (2019).
Korn, T., Bettelli, E., Oukka, M. & Kuchroo, V. K. IL-17 and Th17 cells. Annu Rev. Immunol. 27, 485–517 (2009).
Lai Kwan Lam, Q., King Hung Ko, O., Zheng, B. J. & Lu, L. Local BAFF gene silencing suppresses Th17-cell generation and ameliorates autoimmune arthritis. Proc. Natl Acad. Sci. USA 105, 14993–14998 (2008).
Lin, X. et al. Th17 cells play a critical role in the development of experimental Sjogren’s syndrome. Ann. Rheum. Dis. 74, 1302–1310 (2015).
Patel, D. D., Lee, D. M., Kolbinger, F. & Antoni, C. Effect of IL-17A blockade with secukinumab in autoimmune diseases. Ann. Rheum. Dis. 72, ii116–ii123 (2013).
Amarilyo, G., Lourenco, E. V., Shi, F. D. & La Cava, A. IL-17 promotes murine lupus. J. Immunol. 193, 540–543 (2014).
Turner, J. E. et al. CCR6 recruits regulatory T cells and Th17 cells to the kidney in glomerulonephritis. J. Am. Soc. Nephrol. 21, 974–985 (2010).
Kim, Y. U., Lim, H., Jung, H. E., Wetsel, R. A. & Chung, Y. Regulation of autoimmune germinal center reactions in lupus-prone BXD2 mice by follicular helper T cells. PLoS One 10, e0120294 (2015).
Petri, M. et al. Derivation and validation of the systemic lupus international collaborating clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 64, 2677–2686 (2012).
Streeck, H. et al. Rapid ex vivo isolation and long-term culture of human Th17 cells. J. Immunol. Methods 333, 115–125 (2008).
Fillatreau, S., Sweenie, C. H., McGeachy, M. J., Gray, D. & Anderton, S. M. B cells regulate autoimmunity by provision of IL-10. Nat. Immunol. 3, 944–950 (2002).
Steinmetz, O. M. et al. CXCR3 mediates renal Th1 and Th17 immune response in murine lupus nephritis. J. Immunol. 183, 4693–4704 (2009).
Pawar, R. D. et al. Inhibition of Toll-like receptor-7 (TLR-7) or TLR-7 plus TLR-9 attenuates glomerulonephritis and lung injury in experimental lupus. J. Am. Soc. Nephrol. 18, 1721–1731 (2007).
Austin, H. A. 3rd, Muenz, L. R., Joyce, K. M., Antonovych, T. T. & Balow, J. E. Diffuse proliferative lupus nephritis: identification of specific pathologic features affecting renal outcome. Kidney Int. 25, 689–695 (1984).
Yang, M. et al. IL-10-producing regulatory B10 cells ameliorate collagen-induced arthritis via suppressing Th17 cell generation. Am. J. Pathol. 180, 2375–2385 (2012).
Hartupee, J., Liu, C. N., Novotny, M., Li, X. X. & Hamilton, T. IL-17 enhances chemokine gene expression through mRNA stabilization. J. Immunol. 179, 4135–4141 (2007).
Starke, C. et al. High frequency of autoantibody-secreting cells and long-lived plasma cells within inflamed kidneys of NZB/W F1 lupus mice. Eur. J. Immunol. 41, 2107–2112 (2011).
DiLillo, D. J. et al. Maintenance of long-lived plasma cells and serological memory despite mature and memory B cell depletion during CD20 immunotherapy in mice. J. Immunol. 180, 361–371 (2008).
Tang, H. et al. TLR4 activation is required for IL-17-induced multiple tissue inflammation and wasting in mice. J. Immunol. 185, 2563–2569 (2010).
Reimold, A. M. et al. Plasma cell differentiation requires the transcription factor XBP-1. Nature 412, 300–307 (2001).
Hu, C. C., Dougan, S. K., McGehee, A. M., Love, J. C. & Ploegh, H. L. XBP-1 regulates signal transduction, transcription factors and bone marrow colonization in B cells. EMBO J. 28, 1624–1636 (2009).
Pengo, N. et al. Plasma cells require autophagy for sustainable immunoglobulin production. Nat. Immunol. 14, 298–305 (2013).
Shapiro-Shelef, M. et al. Blimp-1 is required for the formation of immunoglobulin secreting plasma cells and pre-plasma memory B cells. Immunity 19, 607–620 (2003).
Wang, Y. & Bhattacharya, D. Adjuvant-specific regulation of long-term antibody responses by ZBTB20. J. Exp. Med. 211, 841–856 (2014).
Littman, D. R. & Rudensky, A. Y. Th17 and regulatory T cells in mediating and restraining inflammation. Cell 140, 845–858 (2010).
Wu, H. et al. Arginase-1-dependent promotion of TH17 differentiation and disease progression by MDSCs in systemic lupus erythematosus. Sci. Transl. Med. 8, 331ra340 (2016).
Gladiator, A., Wangler, N., Trautwein-Weidner, K. & LeibundGut-Landmann, S. Cutting edge: IL-17-secreting innate lymphoid cells are essential for host defense against fungal infection. J. Immunol. 190, 521–525 (2013).
Shibata, K., Yamada, H., Hara, H., Kishihara, K. & Yoshikai, Y. Resident Vdelta1+ gammadelta T cells control early infiltration of neutrophils after Escherichia coli infection via IL-17 production. J. Immunol. 178, 4466–4472 (2007).
Schlegel, P. M., Steiert, I., Kotter, I. & Muller, C. A. B cells contribute to heterogeneity of IL-17 producing cells in rheumatoid arthritis and healthy controls. PLoS ONE 8, e82580 (2013).
Hirota, K. et al. Autoimmune Th17 cells induced synovial stromal and innate lymphoid cell secretion of the cytokine GM-CSF to initiate and augment autoimmune arthritis. Immunity 48, 1220–1232 e1225 (2018).
Xie, S. et al. IL-17 activates the canonical NF-kappaB signaling pathway in autoimmune B cells of BXD2 mice to upregulate the expression of regulators of G-protein signaling 16. J. Immunol. 184, 2289–2296 (2010).
Lee, S. Y. et al. Inhibition of IL-17 ameliorates systemic lupus erythematosus in Roquin(san/san) mice through regulating the balance of TFH cells, GC B cells, Treg and Breg. Sci. Rep. 9, 5227 (2019).
Mitsdoerffer, M. et al. Proinflammatory T helper type 17 cells are effective B-cell helpers. Proc. Natl Acad. Sci. USA 107, 14292–14297 (2010).
Wang, X. et al. IL-17A promotes pulmonary B-1a cell differentiation via induction of Blimp-1 expression during influenza virus infection. PLoS Pathog. 12, e1005367 (2016).
Lin, X. et al. IL-10-producing regulatory B cells restrain the T follicular helper cell response in primary Sjogren’s syndrome. Cell Mol. Immunol. 16, 921–931 (2019).
Butcher, M. J., Gjurich, B. N., Phillips, T. & Galkina, E. V. The IL-17A/IL-17RA axis plays a proatherogenic role via the regulation of aortic myeloid cell recruitment. Circ. Res. 110, 675–687 (2012).
Taylor, P. R. et al. Activation of neutrophils by autocrine IL-17A-IL-17RC interactions during fungal infection is regulated by IL-6, IL-23, RORgammat and dectin-2. Nat. Immunol. 15, 143–151 (2014).
Goepfert, A., Lehmann, S., Blank, J., Kolbinger, F. & Rondeau, J. M. Structural analysis reveals that the cytokine IL-17F forms a homodimeric complex with receptor IL-17RC to drive IL-17RA-independent signaling. Immunity 52, 499–512 e495 (2020).
Wright, J. F. et al. The human IL-17F/IL-17A heterodimeric cytokine signals through the IL-17RA/IL-17RC receptor complex. J. Immunol. 181, 2799–2805 (2008).
Iwakura, Y., Ishigame, H., Saijo, S. & Nakae, S. Functional specialization of interleukin-17 family members. Immunity 34, 149–162 (2011).
Moseley, T. A., Haudenschild, D. R., Rose, L. & Reddi, A. H. Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev. 14, 155–174 (2003).
Gaffen, S. L. Structure and signalling in the IL-17 receptor family. Nat. Rev. Immunol. 9, 556–567 (2009).
Hedrich, C. M., Rauen, T., Kis-Toth, K., Kyttaris, V. C. & Tsokos, G. C. cAMP-responsive element modulator alpha (CREMalpha) suppresses IL-17F protein expression in T lymphocytes from patients with systemic lupus erythematosus (SLE). J. Biol. Chem. 287, 4715–4725 (2012).
Su, Y. et al. Interleukin-17 receptor D constitutes an alternative receptor for interleukin-17A important in psoriasis-like skin inflammation. Sci. Immunol. 4, eaau9657 (2019).
Zhang, H. et al. IL-17 induces expression of vascular cell adhesion molecule through signalling pathway of NF-kappaB, but not Akt1 and TAK1 in vascular smooth muscle cells. Scand. J. Immunol. 77, 230–237 (2013).
Subramaniam, S. V., Cooper, R. S. & Adunyah, S. E. Evidence for the involvement of JAK/STAT pathway in the signaling mechanism of interleukin-17. Biochem. Biophys. Res. Commun. 262, 14–19 (1999).
Robert, M. & Miossec, P. Interleukin-17 and lupus: enough to be a target? For which patients? Lupus 29, 6–14 (2020).
Acknowledgements
This study was funded by grants from the National Natural Science Foundation of China (Nos. 81771761, 91842304, and 81901635), Chongqing International Institute for Immunology (2020YJC10), and Sanming Project of Medicine in Shenzhen (SZSM201512019). We thank Mr. Otis Ko for the technical support and service of the Medical Faculty Core Facility and Laboratory Animal Unit at The University of Hong Kong. We are grateful to Dr. Yoichiro Iwakura (University of Tokyo) for providing Il17a−/− mice.
Author information
Authors and Affiliations
Contributions
K.M.: experimental design and paper writing; W.D., F.X., E. H., Y.T., C.D., L.L. M.H., Y.C., and S.Y.: mouse experiments and paper preparation; N.P., J.L., D.H., Q.H., X.H., X.C., Q.J., and D.L.: clinical data acquisition and analysis; and L.L.: experimental design, paper preparation, and funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Ma, K., Du, W., Xiao, F. et al. IL-17 sustains the plasma cell response via p38-mediated Bcl-xL RNA stability in lupus pathogenesis. Cell Mol Immunol 18, 1739–1750 (2021). https://doi.org/10.1038/s41423-020-00540-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41423-020-00540-4
Keywords
This article is cited by
-
The IL-17 family in diseases: from bench to bedside
Signal Transduction and Targeted Therapy (2023)
-
B1-cell-produced anti-phosphatidylserine antibodies contribute to lupus nephritis development via TLR-mediated Syk activation
Cellular & Molecular Immunology (2023)
-
Norcantharidin ameliorates the development of murine lupus via inhibiting the generation of IL-17 producing cells
Acta Pharmacologica Sinica (2022)
-
Epigenetic regulation of B cells and its role in autoimmune pathogenesis
Cellular & Molecular Immunology (2022)
-
IL-38, a potential therapeutic agent for lupus, inhibits lupus progression
Inflammation Research (2022)