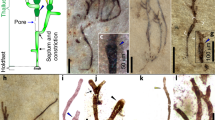
Abstract
Today oxygenic photosynthesis is unique to cyanobacteria and their plastid relatives within eukaryotes. Although its origin before the Great Oxidation Event is still debated1,2,3,4, the accumulation of O2 profoundly modified the redox chemistry of the Earth and the evolution of the biosphere, including complex life. Understanding the diversification of cyanobacteria is thus crucial to grasping the coevolution of our planet and life, but their early fossil record remains ambiguous5. Extant cyanobacteria include the thylakoid-less Gloeobacter-like group and the remainder of cyanobacteria that acquired thylakoid membranes6,7. The timing of this divergence is indirectly estimated at between 2.7 and 2.0 billion years ago (Ga) based on molecular clocks and phylogenies8,9,10,11 and inferred from the earliest undisputed fossil record of Eoentophysalis belcherensis, a 2.018–1.854 Ga pleurocapsalean cyanobacterium preserved in silicified stromatolites12,13. Here we report the oldest direct evidence of thylakoid membranes in a parallel-to-contorted arrangement within the enigmatic cylindrical microfossils Navifusa majensis from the McDermott Formation, Tawallah Group, Australia (1.78–1.73 Ga), and in a parietal arrangement in specimens from the Grassy Bay Formation, Shaler Supergroup, Canada (1.01–0.9 Ga). This discovery extends their fossil record by at least 1.2 Ga and provides a minimum age for the divergence of thylakoid-bearing cyanobacteria at roughly 1.75 Ga. It allows the unambiguous identification of early oxygenic photosynthesizers and a new redox proxy for probing early Earth ecosystems, highlighting the importance of examining the ultrastructure of fossil cells to decipher their palaeobiology and early evolution.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All raw data are deposited in ULiege institutional open archive ORBi and can be accessed at https://hdl.handle.net/2268/308458. The folder ‘morphometry’ contains a table with measurements of microfossil lengths and widths; the folder ‘Raman_RawData’ contains raw maps; the folder ‘Raman_TreatedData_Temperatures’ contains tables with the treated data used to obtain temperatures (palaeothermometry - Raman reflectance T°C Rmc Ro); the folder ‘Raw_TEM_images’ contains raw TEM images of microfossil ultrastructure with scales. All tables are in .txt format.
References
Sánchez-Baracaldo, P., Bianchini, G., Wilson, J. D. & Knoll, A. H. Cyanobacteria and biogeochemical cycles through Earth history. Trends Microbiol. 30, 143–157 (2022).
Ostrander, C. M., Johnson, A. C. & Anbar, A. D. Earth’s first redox revolution. Annu. Rev. Earth Planet. Sci. 49, 337–366 (2021).
Wilmeth, D. T. et al. Evidence for benthic oxygen production in Neoarchean lacustrine stromatolites. Geology 50, 907–911 (2022).
Slotznick, S. P. et al. Reexamination of 2.5-Ga “Whiff” of oxygen interval points to anoxic ocean before GOE. Sci. Adv. 8, eabj7190 (2022).
Demoulin, C. F. et al. Cyanobacteria evolution: insight from the fossil record. Free Rad. Biol. Med. 140, 206–223 (2019).
Rippka, R., Waterbury, J. & Cohen-Bazire, G. A cyanobacterium which lacks thylakoids. Arch. Microbiol. 100, 419–436 (1974).
Komarek, J. & Anagnostidis, K. in Freshwater Flora of Central Europe Vol. 19, (ed. Moltmann, U. G.) 34–36 (Spektrum Akademischer, 2008).
Cavalier-Smith, T. The neomuran origin of archaebacterial, the negibacterial root of the universal tree and bacterial megaclassification. Int. J. Syst. Evol. Microbiol. 52, 7–76 (2002).
Shih, P. M., Hemp, J., Ward, L. M., Matzke, N. J. & Fischer, W. W. Crown group Oxyphotobacteria postdate the rise of oxygen. Geobiology 15, 19–29 (2017).
Rahmatpour, N. et al. A novel thylakoid-less isolate fills a billion-year gap in the evolution of cyanobacteria. Curr. Biol. 31, 2857–2867 (2021).
Fournier, G. P. et al. The Archean origin of oxygenic photosynthesis and extant cyanobacterial lineages. Proc. R. Soc. Lond. B Biol. Sci. 288, 20210675 (2021).
Hofmann, H. J. Precambrian microflora, Belcher Islands, Canada: significance and systematics. J. Paleontol. 50, 1040–1073 (1976).
Hodgskiss, M. S. et al. New insights on the Orosirian carbon cycle, early Cyanobacteria, and the assembly of Laurentia from the Paleoproterozoic Belcher Group. Earth Planet. Sci. Lett. 520, 141–152 (2019).
Jabłońska, J. & Tawfik, D. S. The evolution of oxygen-utilizing enzymes suggests early biosphere oxygenation. Nat. Ecol. Evol. 5, 442–448 (2021).
Cardona, T., Sánchez-Baracaldo, P., Rutherford, A. W. & Larkum, A. W. D. Early Archean origin of Photosystem II. Geobiology 17, 127–150 (2019).
Sánchez-Baracaldo, P. & Cardona, T. On the origin of oxygenic photosynthesis and cyanobacteria. New Phytol. 225, 1440–1446 (2020).
Blank, C. E. & Sánchez-Baracaldo, P. Timing of morphological and ecological innovations in the cyanobacteria a key to understand the rise in atmospheric oxygen. Geobiology 8, 1–23 (2010).
Schirrmeister, B. E., Gugger, M. & Donoghue, P. C. Cyanobacteria and the Great Oxidation Event: evidence from genes and fossils. Palaeontology 58, 769–785 (2015).
Shih, P. M. et al. Biochemical characterization of predicted Precambrian RuBisCO. Nat. Commun. 7, 10382 (2016).
Schwartz, R. M. & Dayhoff, M. O. Origins of prokaryotes, eukaryotes, mitochondria, and chloroplasts. Science 199, 395–403 (1978).
Golubic, S. & Hofmann, H. J. Comparison of Holocene and mid-Precambrian Entophysalidaceae (Cyanophyta) in stromatolitic algal mats: cell division and degradation. J. Paleontol. 50, 1074–1082 (1976).
Butterfield, N. J. Proterozoic photosynthesis – a critical review. Palaeontology 58, 953–972 (2015).
Sergeev, V. N. Microfossils in cherts from the middle riphean (mesoproterozoic) Avzyan Formation, southern ural Mountains, Russian federation. Precambrian Res. 65, 231–254 (1994).
Zhang, Y. Proterozoic stromatolitic micro-organisms from Hebei, North China: cell preservation and cell division. Precambrian Res. 38, 165–175 (1988).
Javaux, E. J., Knoll, A. H. & Walter, M. R. TEM evidence for eukaryotic diversity in mid-Proterozoic oceans. Geobiology 2, 121–132 (2004).
Loron, C. C., Rainbird, R. H., Turner, E. C., Greenman, J. W. & Javaux, E. J. Organic-walled microfossils from the late Mesoproterozoic to early Neoproterozoic lower Shaler Supergroup (Arctic Canada): diversity and biostratigraphic significance. Precambrian Res. 321, 349–374 (2019).
Shimoni, E., Rav-Hon, O., Ohad, I., Brumfeld, V. & Reich, Z. Three-dimensional organization of higher-plant chloroplast thylakoid membranes revealed by electron tomography. Plant Cell 17, 2580–2586 (2005).
Gonzalez-Esquer, C. R. et al. Cyanobacterial ultrastructure in light of genomic sequence data. Photosynth. Res. 129, 147–157 (2016).
Mareš, J., Strunecký, O., Bučinská, L. & Wiedermannova, J. Evolutionary patterns of thylakoid architecture in cyanobacteria. Front. Microbiol. 10, 277 (2019).
Mareš, J. et al. The primitive thylakoid-less cyanobacterium Gloeobacter is a common rock-dwelling organism. PLoS ONE 8, e66323 (2013).
Nelissen, B., Van de Peer, Y., Wilmotte, A. & De Wachter, R. An early origin of platids within the cyanobacterial divergence is suggested by evolutionary trees based on complete 16S rRNA sequences. Mol. Biol. Evol. 12, 1166–1173 (1995).
Raven, J. A. & Sànchez-Baracaldo, P. Gloeobacter and the implications of a freshwater origin of cyanobacteria. Phycologia 60, 402–418 (2021).
Guéguen, N. & Maréchal, E. Origin of cyanobacterial thylakoids via a non-vesicvular glycolipid phase transition and their impact on the Great Oxygenation Event. J. Exp. Bot. 73, 2721–2734 (2022).
Pacton, M., Gorin, G. E. & Fiet, N. Unravelling the origin of ultralaminae in sedimentary organic matter: the contribution of bacteria and photosynthetic organisms. J. Sediment. Res. 78, 654–667 (2008).
Kremer, B., Kaźmierczak, J. & Środoń, J. Cyanobacterial-algal crusts from Late Ediacaran paleosols of the East European Craton. Precambrian Res. 305, 236–246 (2018).
Schoenhut, K., Vann, D. R. & LePage, B. A. Cytological and ultrastructural preservation in Eocene Metasequoia leaves from the Canadian High Arctic. Am. J. Bot. 91, 816–824 (2004).
Wang, X., Liu, W., Du, K., He, X. & Jin, J. Ultrastructural of chloroplasts in fossil Nelumbo from the Eocene of Hainan Island, South China. Plant Syst. Evol. 300, 2259–2264 (2014).
Lepot, K. et al. Organic and mineral imprints in fossil photosynthetic mats of an East-Antarctic lake. Geobiol. 12, 424–450 (2014).
Miao, L., Moczydłowska, M., Zhu, S. & Zhu, M. New record of organic-walled, morphologically distinct microfossils from the late Paleoproterozoic ChangCheng Group in the Yanshan Range, North China. Precambrian Res. 321, 172–198 (2019).
Spinks, S. C., Schmid, S. & Pagès, A. Delayed euxinia in Paleoproterozoic intracontinental seas: vital havens for the evolution of eukaryotes. Precambrian Res. 287, 108–114 (2016).
François, C. et al. Multi-method dating constrains the diversification of early 2 eukaryotes in the Proterozoic Mbuji-Mayi Supergroup of the D.R.Congo and the geological evolution of the Congo Basin. J. Afr. Earth Sci. 198, 104785 (2023).
Baludikay, B. K., Storme, J. Y., François, C., Baudet, D. & Javaux, E. J. A diverse and exquisitely preserved organic-walled microfossil assemblage from the Meso–Neoproterozoic Mbuji-Mayi Supergroup (Democratic Republic of Congo) and implications for Proterozoic biostratigraphy. Precambrian Res. 281, 166–18 (2016).
Pyatiletov, V. G. Yudoma complex microfossils from southern Yakutia. Geol. Geofiz. 7, 8–20 (1980).
Hofmann, H. J. & Jackson, G. D. Shale-facies microfossils from the Proterozoic Bylot Supergroup, Baffin Island, Canada. J. Paleontol. 68, 1–35 (1994).
Kirchhoff, H. Chloroplast ultrastructure in plants. New Phytol. 223, 565–574 (2019).
Meng, L. et al. Measuring the dynamic response of the thylakoid architecture in plant leaves by electron microscopy. Plant Direct. 4, e00280 (2020).
Spinks, S. C., Schmid, S., Pagés, A. & Bluett, J. Evidence for SEDEX-style mineralization in the 1.7 Ga Tawallah Group, McArthur basin, Australia. Ore Geol. Rev. 76, 122–139 (2018).
Javaux, E. J., Marshall, C. P. & Bekker, A. Organic-walled microfossils in 3.2-billion-year-old shallow-marine siliciclastic deposits. Nature 463, 934–938 (2010).
Fatka, O. & Brocke, R. Morphological variability and method of opening of the Devonian acritarch Navifusa bacilla. Rev. Palaeobot. Palynol. 148, 108–123 (2008).
Horodyski, R. J. & Donaldson, J. A. Microfossils from the middle Proterozoic Dismal Lakes Groups, Arctic Canada. Precambrian Res. 11, 125–159 (1980).
Golubic, S., Sergeev, V. N. & Knoll, A. H. Mesoproterozoic Archaeoellipsoides: akinetes of heterocystous cyanobacteria. Lethaia 28, 285–298 (1995).
Tomitani, A., Knoll, A. H., Cavanaugh, C. M. & Ohno, T. The evolutionary diversification of cyanobacteria: molecular–phylogenetic and paleontological perspectives. Proc. Natl Acad. Sci. USA 103, 5442–5447 (2006).
Kaplan-Levy, R. N., Hadas, O., Summers, M. L., Rücker, J. & Sukenik, A. in Dormancy and Resistance in Harsh Environments (eds Lubzens, E. et al.) 5–27 (Springer, 2010).
Sergeev, V. N., Knoll, A. H., Vorob’eva, N. G. & Sergeeva, N. D. Microfossils from the lower Mesoproterozoic Kaltasy Formation, East European Platform. Precambrian Res. 278, 87–107 (2015).
Sukenik, A., Rücker, J. & Maldener, I. in Cyanobacteria from Basic Science to Applications (eds Mishra, A. K. et al.) 65–77 (Academic, 2019).
Perez, R., Forchhammer, K., Salerno, G. & Maldener, I. Clear differences in metabolic and porphological adaptations of akinetes of two Nostocales living in different habitats. Microbiology 162, 214–223 (2016).
López-García, P. & Moreira, D. The Syntrophy hypothesis for the origin of eukaryotes revisited. Nat. Microbiol. 5, 655–667 (2020).
Javaux, E. J. in Encyclopedia of Astrobiology (eds Gargaud, M. et al.), Ch. 538–4, 1–5 (Springer, 2021).
Baludikay, B. K. et al. Raman microspectroscopy, bitumen reflectance and illite crystallinity scale: comparison of different geothermometry methods on fossiliferous Proterozoic sedimentary basins (DR Congo, Mauritania and Australia). Int. J. Coal Geol. 191, 80–94 (2018).
Grey, K. A modified palynological preparation technique for the extraction of large Neoproterozoic acanthomorph acritarchs and other acid-insoluble microfossils. Western Australia Geological Survey, Record 1999/10 (1999).
Acknowledgements
We thank the Royal Museum for Central Africa (Tervuren, Belgium) and D. Baudet for access to the Kanshi SB13 drill core; S. Spinks and M. Kunzmann (CSIRO Mineral Resources, Australia) for samples from the GSD7 drill core at the Darwin core facility (Australia); and the Geological Survey of Canada’s Geomapping for Energy and Minerals programme, G. Halverson (McGill University, Canada), R. Rainbird (GSC, Canada), E. Turner (Laurentian University, Canada), T. Gibson (McGill University, Canada) and C. Loron (ULiege, Belgium and University of Edinburgh, UK) for sampling the Shaler Supergroup in the Northwest Territories of Arctic Canada. We thank M. Giraldo at the Early Life Traces & Evolution–Astrobiology laboratory and C. López-Iglesias and H. Duimel at the Microscopy CORE Lab (University of Maastricht) for technical support. FRS-FNRS-FWO EOS ET-Home (grant no. 30442502), ERC Stg ELiTE FP7/308074, an Agouron Institute geobiology grant and BELSPO BRAIN project B2/212/PI/PORTAL supported this project.
Author information
Authors and Affiliations
Contributions
C.F.D., Y.J.L. and E.J.J. conceived the study and interpreted the data. A.L. performed acid demineralization and prepared microfossil slides. C.F.D. and E.J.J. performed TEM sample preparation and observations. C.F.D. and E.J.J. prepared samples for Raman spectroscopy. C.F.D., Y.J.L. and E.J.J. performed Raman analyses. C.F.D., Y.J.L. and E.J.J. wrote the paper. E.J.J. supervised the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Helmut Kirchhoff and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 TEM pictures of a specimen of Navifusa majensis, from the Grassy Bay Formation (Shaler Supergroup, Canada).
Pictures a, c and d show the width and limits of each layer interpreted as stacked thylakoidal membranes that were measured. Measurements are compiled in Extended Data Table 1 below. Picture b is a zoom of the section through the microfossil rounded end in a (black box). The parietal arrangement is clearly visible (dotted black lines), as well as the variable thicknesses of stacked thylakoidal membranes, due to merging of several thylakoids during burial, compression and diagenesis, dotted yellow lines show possible limits of several layers in the ticker one. These TEM pictures are from the same specimen illustrated in Fig. 2c,d; 3a,b.
Extended Data Fig. 2 TEM pictures of the second specimen of Navifusa majensis from the Grassy Bay Formation (Shaler Supergroup, Canada).
Pictures a and b show some positions where thickness of layers were measured and clearly illustrate the limits of each layer interpreted as stacked thylakoidal membranes. Measures are summarized in the Extended Data Table 1 below. Picture c shows knife marks (dotted lines) creating artefacts on ultrathin sections. These knife marks are clearly distinguishable from limits of stacked thylakoidal layers. Picture c also shows that on a same ultrathin section, the limits between layers may be less clear, due to merging during burial and compression. n = 2 N. majensis for Grassy Bay Formation. “n” represents the number of specimens observed by TEM.
Extended Data Fig. 3 TEM picture of a specimen of Navifusa majensis from the McDermott Formation (Tawallah Group, Australia).
This picture shows the positions where the thylakoidal membranes are measured. Measurements are compiled in table S4 below. n = 2 N. majensis for McDermott Formation. “n” represents the number of specimens observed by TEM.
Extended Data Fig. 4 Schematic drawings showing how compressed microfossils were cut transversally for TEM observations.
a represents a whole flattened specimen of Navifusa majensis, with transversal section shown (black line). b represents a transversal TEM ultrathin section through the microfossil. The length of the ultrathin section in b corresponds to the width of the microfossil (red line in a and b), while the thickness of the ultrathin section corresponds to the thickness of the compressed microfossil (T in a and b). L: length of the whole microfossil; W: width; T: thickness.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Demoulin, C.F., Lara, Y.J., Lambion, A. et al. Oldest thylakoids in fossil cells directly evidence oxygenic photosynthesis. Nature 625, 529–534 (2024). https://doi.org/10.1038/s41586-023-06896-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-06896-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.