Abstract
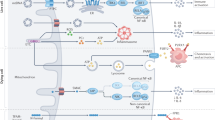
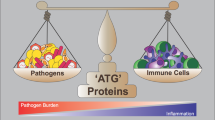
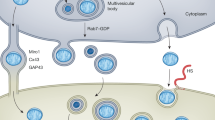
Mitochondria retain bacterial traits due to their endosymbiotic origin, but host cells do not recognize them as foreign because the organelles are sequestered. However, the regulated release of mitochondrial factors into the cytosol can trigger cell death, innate immunity and inflammation. This selective breakdown in the 2-billion-year-old endosymbiotic relationship enables mitochondria to act as intracellular signalling hubs. Mitochondrial signals include proteins, nucleic acids, phospholipids, metabolites and reactive oxygen species, which have many modes of release from mitochondria, and of decoding in the cytosol and nucleus. Because these mitochondrial signals probably contribute to the homeostatic role of inflammation, dysregulation of these processes may lead to autoimmune and inflammatory diseases. A potential reason for the increased incidence of these diseases may be changes in mitochondrial function and signalling in response to such recent phenomena as obesity, dietary changes and other environmental factors. Focusing on the mixed heritage of mitochondria therefore leads to predictions for future insights, research paths and therapeutic opportunities. Thus, whereas mitochondria can be considered ‘the enemy within’ the cell, evolution has used this strained relationship in intriguing ways, with increasing evidence pointing to the recent failure of endosymbiosis being critical for the pathogenesis of inflammatory diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Murphy, M. P. & Hartley, R. C. Mitochondria as a therapeutic target for common pathologies. Nat. Rev. Drug Discov. 17, 865–886 (2018). The many pathogical roles of mitochondria are discussed.
Chandel, N. S. Evolution of mitochondria as signaling organelles. Cell Metab. 22, 204–206 (2015). The key signalling roles of mitochondria are discussed in an evolutionary context.
Picard, M. & Shirihai, O. S. Mitochondrial signal transduction. Cell Metab. 34, 1620–1653 (2022).
Monzel, A. S., Enriquez, J. A. & Picard, M. Multifaceted mitochondria: moving mitochondrial science beyond function and dysfunction. Nat. Metab. 5, 546–562 (2023). A recent review that highlights the many emerging facets of mitochondrial biology.
Tait, S. W. & Green, D. R. Mitochondria and cell signalling. J. Cell Sci. 125, 807–815 (2012).
Bahat, A., MacVicar, T. & Langer, T. Metabolism and innate immunity meet at the mitochondria. Front. Cell Dev. Biol. 9, 720490 (2021).
Marchi, S., Guilbaud, E., Tait, S. W. G., Yamazaki, T. & Galluzzi, L. Mitochondrial control of inflammation. Nat. Rev. Immunol. 23, 159–173 (2023).
Wein, T. & Sorek, R. Bacterial origins of human cell-autonomous innate immune mechanisms. Nat. Rev. Immunol. 22, 629–638 (2022). Here the authors suggest how the origins of mitochondria can lead to innate immunity mechanisms.
Krysko, D. V. et al. Emerging role of damage-associated molecular patterns derived from mitochondria in inflammation. Trends Immunol. 32, 157–164 (2011).
Galluzzi, L., Kepp, O. & Kroemer, G. Mitochondria: master regulators of danger signalling. Nat. Rev. Mol. Cell Biol. 13, 780–788 (2012).
Kaplan, G. G. & Windsor, J. W. The four epidemiological stages in the global evolution of inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 18, 56–66 (2021).
Dinse, G. E. et al. Increasing prevalence of antinuclear antibodies in the United States. Arthritis Rheumatol. 72, 1026–1035 (2020).
Wang, R., Li, Z., Liu, S. & Zhang, D. Global, regional and national burden of inflammatory bowel disease in 204 countries and territories from 1990 to 2019: a systematic analysis based on the Global Burden of Disease Study 2019. BMJ Open 13, e065186 (2023).
Duarte-Garcia, A. et al. Rising incidence and prevalence of systemic lupus erythematosus: a population-based study over four decades. Ann. Rheum. Dis. https://doi.org/10.1136/annrheumdis-2022-222276 (2022).
Walton, C. et al. Rising prevalence of multiple sclerosis worldwide: insights from the Atlas of MS, third edition. Mult. Scler. 26, 1816–1821 (2020).
Shi, G. et al. Estimation of the global prevalence, incidence, years lived with disability of rheumatoid arthritis in 2019 and forecasted incidence in 2040: results from the Global Burden of Disease Study 2019. Clin. Rheumatol. 42, 2297–2309 (2023).
Furman, D. et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 25, 1822–1832 (2019). An excellent account of how chronic inflammation changes as we age and how environmental factors impact on inflammatory diseases.
Gontier, N. in Encyclopedia of Evolutionary Biology Vol. 4 (ed. Kliman, R. M.) 261–271 (Elsevier, 2016).
Garg, S., Zimorski, V. & Martin, W. F. in Encyclopedia of Evolutionary Biology Vol. 1 (ed. Kliman, R. M.) 511–517 (Elsevier, 2016).
Dacks, J. B. et al. The changing view of eukaryogenesis – fossils, cells, lineages and how they all come together. J. Cell Sci. 129, 3695–3703 (2016).
Roger, A. J., Munoz-Gomez, S. A. & Kamikawa, R. The origin and diversification of mitochondria. Curr. Biol. 27, R1177–R1192 (2017).
Sagan, L. On the origin of mitosing cells. J. Theor. Biol. 14, 255–274 (1967). A classic paper that led to the acceptance of endosymbiosis as the origin of mitochondria.
Zaremba-Niedzwiedzka, K. et al. Asgard archaea illuminate the origin of eukaryotic cellular complexity. Nature 541, 353–358 (2017).
Martin, W. F. & Mentel, M. The origin of mitochondria. Nat. Educ. 3, 58 (2010).
John, P. & Whatley, F. R. Paracoccus denitrificans and the evolutionary origin of the mitochondrion. Nature 254, 495–498 (1975).
Geiger, O., Sanchez-Flores, A., Padilla-Gomez, J. & Degli Esposti, M. Multiple approaches of cellular metabolism define the bacterial ancestry of mitochondria. Sci. Adv. 9, eadh0066 (2023).
Martin, W. & Muller, M. The hydrogen hypothesis for the first eukaryote. Nature 392, 37–41 (1998).
Raval, P. K., Martin, W. F. & Gould, S. B. Mitochondrial evolution: gene shuffling, endosymbiosis, and signaling. Sci. Adv. 9, eadj4493 (2023).
Wallace, D. C. Mitochondrial diseases in man and mouse. Science 283, 1482–1488 (1999).
Gorman, G. S. et al. Mitochondrial diseases. Nat. Rev. Dis. Primers 2, 16080 (2016).
Gustafsson, C. M., Falkenberg, M. & Larsson, N. G. Maintenance and expression of mammalian mitochondrial DNA. Annu. Rev. Biochem. 85, 133–160 (2016).
Rath, S. et al. MitoCarta3.0: an updated mitochondrial proteome now with sub-organelle localization and pathway annotations. Nucleic Acids Res. 49, D1541–D1547 (2021).
Morgenstern, M. et al. Quantitative high-confidence human mitochondrial proteome and its dynamics in cellular context. Cell Metab. 33, 2464–2483 (2021).
Gross, J. & Bhattacharya, D. Mitochondrial and plastid evolution in eukaryotes: an outsiders’ perspective. Nat. Rev. Genet. 10, 495–505 (2009).
Paschen, S. A., Neupert, W. & Rapaport, D. Biogenesis of beta-barrel membrane proteins of mitochondria. Trends Biochem. Sci. 30, 575–582 (2005).
Gross, A. et al. Caspase cleaved BID targets mitochondria and is required for cytochrome c release, while Bcl-Xl prevents this release but not tumor necrosis factor-R1/Fas death. J. Biol. Chem. 274, 1156–1163 (1999).
Liu, X. S., Kim, C. N., Yang, J., Jemmerson, R. & Wang, X. D. Induction of apoptotic program in cell-free extracts – requirement for datp and cytochrome c. Cell 86, 147–157 (1996).
Giacomello, M., Pyakurel, A., Glytsou, C. & Scorrano, L. The cell biology of mitochondrial membrane dynamics. Nat. Rev. Mol. Cell Biol. 21, 204–224 (2020).
Kalkavan, H. & Green, D. R. MOMP, cell suicide as a BCL-2 family business. Cell Death Differ. 25, 46–55 (2018).
Suhaili, S. H., Karimian, H., Stellato, M., Lee, T. H. & Aguilar, M. I. Mitochondrial outer membrane permeabilization: a focus on the role of mitochondrial membrane structural organization. Biophys. Rev. 9, 443–457 (2017).
He, B. et al. Mitochondrial cristae architecture protects against mtDNA release and inflammation. Cell Rep. 41, 111774 (2022).
Frezza, C. et al. OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell 126, 177–189 (2006).
Ott, M., Zhivotovsky, B. & Orrenius, S. Role of cardiolipin in cytochrome c release from mitochondria. Cell Death Differ. 14, 1243–1247 (2007).
Munoz-Gomez, S. A., Slamovits, C. H., Dacks, J. B. & Wideman, J. G. The evolution of MICOS: ancestral and derived functions and interactions. Commun. Integr. Biol. 8, e1094593 (2015).
Friedman, J. R., Mourier, A., Yamada, J., McCaffery, J. M. & Nunnari, J. MICOS coordinates with respiratory complexes and lipids to establish mitochondrial inner membrane architecture. eLife 4, e07739 (2015).
Bernardi, P. & Di Lisa, F. The mitochondrial permeability transition pore: molecular nature and role as a target in cardioprotection. J. Mol. Cell. Cardiol. 78, 100–106 (2015).
Scarlett, J. L. & Murphy, M. P. Release of apoptogenic proteins from the mitochondrial intermembrane space during the mitochondrial permeability transition. FEBS Lett. 418, 282–286 (1997).
Bernardi, P. et al. Identity, structure, and function of the mitochondrial permeability transition pore: controversies, consensus, recent advances, and future directions. Cell Death Differ. 30, 1869–1885 (2023).
Liu, B. et al. CpG methylation patterns of human mitochondrial DNA. Sci. Rep. 6, 23421 (2016).
Riley, J. S. & Tait, S. W. Mitochondrial DNA in inflammation and immunity. EMBO Rep. 21, e49799 (2020).
Kim, J., Kim, H. S. & Chung, J. H. Molecular mechanisms of mitochondrial DNA release and activation of the cGAS-STING pathway. Exp. Mol. Med. 55, 510–519 (2023).
McArthur, K. et al. BAK/BAX macropores facilitate mitochondrial herniation and mtDNA efflux during apoptosis. Science 359, 6378 (2018). First description of a role for BAK/BAX and mitochondrial herniation in the release of mtDNA.
Kim, J. et al. VDAC oligomers form mitochondrial pores to release mtDNA fragments and promote lupus-like disease. Science 366, 1531–1536 (2019). Evidence for oxidized mtDNA as an activator of the NLRP3 inflammasome.
Xian, H. et al. Oxidized DNA fragments exit mitochondria via mPTP- and VDAC-dependent channels to activate NLRP3 inflammasome and interferon signaling. Immunity 55, 1370–1385 (2022). Evidence for oxidized mtDNA as an activator of the NLRP3 inflammasome.
Mills, E. L., Kelly, B. & O’Neill, L. A. J. Mitochondria are the powerhouses of immunity. Nat. Immunol. 18, 488–498 (2017).
Billingham, L. K. et al. Mitochondrial electron transport chain is necessary for NLRP3 inflammasome activation. Nat. Immunol. 23, 692–704 (2022). Evidence that mitochondrial phosphocreatine generated from ATP derived from oxidative phosphorylation is required for ATP production in the cytosol by creatine kinase B, for NLRP3 activation.
Chowdhury, A., Witte, S. & Aich, A. Role of mitochondrial nucleic acid sensing pathways in health and patho-physiology. Front. Cell Dev. Biol. 10, 796066 (2022).
Chen, Y. G. & Hur, S. Cellular origins of dsRNA, their recognition and consequences. Nat. Rev. Mol. Cell Biol. 23, 286–301 (2022).
Seth, R. B., Sun, L., Ea, C. K. & Chen, Z. J. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell 122, 669–682 (2005).
Wang, F., Zhang, D., Zhang, D., Li, P. & Gao, Y. Mitochondrial protein translation: emerging roles and clinical significance in disease. Front. Cell Dev. Biol. 9, 675465 (2021).
Walker, J. E., Carroll, J., Altman, M. C. & Fearnley, I. M. Chapter 6 mass spectrometric characterization of the thirteen subunits of bovine respiratory complexes that are encoded in mitochondrial DNA. Methods Enzymol. 456, 111–131 (2009).
Le, Y., Murphy, P. M. & Wang, J. M. Formyl-peptide receptors revisited. Trends Immunol. 23, 541–548 (2002).
Dorward, D. A. et al. Novel role for endogenous mitochondrial formylated peptide-driven formyl peptide receptor 1 signalling in acute respiratory distress syndrome. Thorax 72, 928–936 (2017).
Cai, N. et al. Mitochondrial DNA variants modulate N-formylmethionine, proteostasis and risk of late-onset human diseases. Nat. Med. 27, 1564–1575 (2021). A fascinating report linking mitochondrial N-formylmethionine formation and pathology.
Paradies, G., Paradies, V., Ruggiero, F. M. & Petrosillo, G. Role of cardiolipin in mitochondrial function and dynamics in health and disease: molecular and pharmacological aspects. Cells 8, 728 (2019).
Pizzuto, M. & Pelegrin, P. Cardiolipin in immune signaling and cell death. Trends Cell Biol. 30, 892–903 (2020).
Dudek, J. Role of cardiolipin in mitochondrial signaling pathways. Front. Cell Dev. Biol. 5, 90 (2017).
Iyer, S. S. et al. Mitochondrial cardiolipin is required for Nlrp3 inflammasome activation. Immunity 39, 311–323 (2013).
Matzinger, P. Tolerance, danger, and the extended family. Annu. Rev. Immunol. 12, 991–1045 (1994).
Murphy, M. P. How mitochondria produce reactive oxygen species. Biochem. J 417, 1–13 (2009). An overview of how mitochondrial redox signals may be generated.
Wong, H. S., Dighe, P. A., Mezera, V., Monternier, P. A. & Brand, M. D. Production of superoxide and hydrogen peroxide from specific mitochondrial sites under different bioenergetic conditions. J. Biol. Chem. 292, 16804–16809 (2017).
Robb, E. L. et al. Control of mitochondrial superoxide production by reverse electron transport at complex I. J. Biol. Chem. 293, 9869–9879 (2018).
Wright, J. J. et al. Reverse electron transfer by respiratory complex I catalyzed in a modular proteoliposome system. J. Am. Chem. Soc. 144, 6791–6801 (2022).
Roca, F. J., Whitworth, L. J., Prag, H. A., Murphy, M. P. & Ramakrishnan, L. Tumor necrosis factor induces pathogenic mitochondrial ROS in tuberculosis through reverse electron transport. Science. 376, eabh2841 (2022).
Murphy, M. P. & Chouchani, E. T. Why succinate? Physiological regulation by a mitochondrial coenzyme Q sentinel. Nat. Chem. Biol. 18, 461–469 (2022).
Mills, E. L. et al. Succinate dehydrogenase supports metabolic repurposing of mitochondria to drive inflammatory macrophages. Cell 167, 457–470 (2016). This paper describes how mitochondrial metabolism can be repurposed to generate succinate as a signal.
Xiao, H. et al. A quantitative tissue-specific landscape of protein redox regulation during aging. Cell 180, 968–983 (2020).
Holmstrom, K. M. & Finkel, T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat. Rev. Mol. Cell Biol. 15, 411–421 (2014).
Christman, M. F., Storz, G. & Ames, B. N. OxyR, a positive regulator of hydrogen peroxide-inducible genes in Escherichia coli and Salmonella typhimurium, is homologous to a family of bacterial regulatory proteins. Proc. Natl Acad. Sci. USA 86, 3484–3488 (1989).
Redza-Dutordoir, M. & Averill-Bates, D. A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta 1863, 2977–2992 (2016).
West, A. P. et al. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature 472, 476–480 (2011).
Ryan, D. G. et al. Coupling Krebs cycle metabolites to signalling in immunity and cancer. Nat. Metab. 1, 16–33 (2019).
Murphy, M. P. & O’Neill, L. A. J. Krebs cycle reimagined: the emerging roles of succinate and itaconate as signal transducers. Cell 174, 780–784 (2018).
Martinez-Reyes, I. & Chandel, N. S. Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun. 11, 102 (2020).
Sivanand, S., Viney, I. & Wellen, K. E. Spatiotemporal control of acetyl-CoA metabolism in chromatin regulation. Trends Biochem. Sci. 43, 61–74 (2018).
Tannahill, G. M. et al. Succinate is an inflammatory signal that induces IL-1beta through HIF-1alpha. Nature 496, 238–242 (2013). Evidence for macrophage-derived succinate being a pro-inflammatory signal.
Selak, M. A. et al. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell 7, 77–85 (2005). A key paper linking succinate to HIF1-alpha activation.
Matilainen, O., Quiros, P. M. & Auwerx, J. Mitochondria and epigenetics – crosstalk in homeostasis and stress. Trends Cell Biol. 27, 453–463 (2017).
Santos, J. H. Mitochondria signaling to the epigenome: a novel role for an old organelle. Free Radic. Biol. Med. 170, 59–69 (2021).
Mills, E. L. et al. Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature 556, 113–117 (2018).
Day, E. A. & O’Neill, L. A. J. Protein targeting by the itaconate family in immunity and inflammation. Biochem. J. 479, 2499–2510 (2022).
McGettrick, A. F. & O’Neill, L. A. Two for the price of one: itaconate and its derivatives as an anti-infective and anti-inflammatory immunometabolite. Curr. Opin. Immunol. 80, 102268 (2023).
DeBerardinis, R. J. & Chandel, N. S. Fundamentals of cancer metabolism. Sci. Adv. 2, e1600200 (2016).
Liberti, M. V. & Locasale, J. W. The Warburg effect: how does it benefit cancer cells? Trends Biochem. Sci. 41, 211–218 (2016).
DeBerardinis, R. J. & Chandel, N. S. We need to talk about the Warburg effect. Nat. Metab. 2, 127–129 (2020).
Weinberg, F. et al. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc. Natl Acad. Sci. USA 107, 8788–8793 (2010).
Martinez-Reyes, I. et al. Mitochondrial ubiquinol oxidation is necessary for tumour growth. Nature 585, 288–292 (2020).
Sena, L. A. et al. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity 38, 225–236 (2013).
Frenkel-Pinter, M. et al. Adaptation and exaptation: from small molecules to feathers. J. Mol. Evol. 90, 166–175 (2022).
Jarman, O. D., Biner, O., Wright, J. J. & Hirst, J. Paracoccus denitrificans: a genetically tractable model system for studying respiratory complex I. Sci. Rep. 11, 10143 (2021).
Henry, M. F. & Vignais, P. M. Production of superoxide anions in Paracoccus denitrificans. Arch. Biochem. Biophys. 203, 365–371 (1980).
Kotlyar, A. B. & Borovok, N. NADH oxidation and NAD+ reduction catalysed by tightly coupled inside-out vesicles from Paracoccus denitrificans. Eur. J. Biochem. 269, 4020–4024 (2002).
Hong, Y., Zeng, J., Wang, X., Drlica, K. & Zhao, X. Post-stress bacterial cell death mediated by reactive oxygen species. Proc. Natl Acad. Sci. USA 116, 10064–10071 (2019).
Lopatina, A., Tal, N. & Sorek, R. Abortive infection: bacterial suicide as an antiviral immune strategy. Annu. Rev. Virol. 7, 371–384 (2020).
Toyofuku, M., Schild, S., Kaparakis-Liaskos, M. & Eberl, L. Composition and functions of bacterial membrane vesicles. Nat. Rev. Microbiol. 21, 415–430 (2023).
Horvath, P. & Barrangou, R. CRISPR/Cas, the immune system of bacteria and archaea. Science 327, 167–170 (2010).
Georjon, H. & Bernheim, A. The highly diverse antiphage defence systems of bacteria. Nat. Rev. Microbiol. 21, 686–700 (2023).
Li, Y. et al. cGLRs are a diverse family of pattern recognition receptors in innate immunity. Cell 186, 3261–3276 (2023).
Wu, X. et al. Phylogenetic and molecular evolutionary analysis of mitophagy receptors under hypoxic conditions. Front. Physiol. 8, 539 (2017).
Zhang, Q. et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 464, 104–107 (2010).
Moriyama, M., Koshiba, T. & Ichinohe, T. Influenza A virus M2 protein triggers mitochondrial DNA-mediated antiviral immune responses. Nat. Commun. 10, 4624 (2019). A role for mitochondrial DNA in the induction of anti-viral immunity in response to an RNA virus (influenza).
Jahun, A. S. et al. Leaked genomic and mitochondrial DNA contribute to the host response to noroviruses in a STING-dependent manner. Cell Rep. 42, 112179 (2023).
Sun, B. et al. Dengue virus activates cGAS through the release of mitochondrial DNA. Sci. Rep. 7, 3594 (2017).
West, A. P. et al. Mitochondrial DNA stress primes the antiviral innate immune response. Nature 520, 553–557 (2015).
Colaco, C. B., Scadding, G. K. & Lockhart, S. Anti-cardiolipin antibodies in neurological disorders: cross-reaction with anti-single stranded DNA activity. Clin. Exp. Immunol. 68, 313–319 (1987).
Colapietro, F., Lleo, A. & Generali, E. Antimitochondrial antibodies: from bench to bedside. Clin. Rev. Allergy Immunol. 63, 166–177 (2022).
Chen, P. M. & Tsokos, G. C. Mitochondria in the pathogenesis of systemic lupus erythematosus. Curr. Rheumatol. Rep. 24, 88–95 (2022).
Becker, Y. et al. Autoantibodies in systemic lupus erythematosus target mitochondrial RNA. Front. Immunol. 10, 1026 (2019).
Chouchani, E. T. et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 515, 431–435 (2014).
Littlewood-Evans, A. et al. GPR91 senses extracellular succinate released from inflammatory macrophages and exacerbates rheumatoid arthritis. J. Exp. Med. 213, 1655–1662 (2016).
Li, Q. et al. RNA editing underlies genetic risk of common inflammatory diseases. Nature 608, 569–577 (2022).
Hooftman, A. et al. Macrophage fumarate hydratase restrains mtRNA-mediated interferon production. Nature 615, 490–498 (2023).
Zecchini, V. et al. Fumarate induces vesicular release of mtDNA to drive innate immunity. Nature 615, 499–506 (2023).
Sciacovelli, M. et al. Fumarate is an epigenetic modifier that elicits epithelial-to-mesenchymal transition. Nature 537, 544–547 (2016).
Whiteley, M., Diggle, S. P. & Greenberg, E. P. Progress in and promise of bacterial quorum sensing research. Nature 551, 313–320 (2017).
Lopez-Domenech, G. et al. Miro proteins coordinate microtubule- and actin-dependent mitochondrial transport and distribution. EMBO J. 37, 321–336 (2018).
Debattisti, V., Gerencser, A. A., Saotome, M., Das, S. & Hajnoczky, G. ROS control mitochondrial motility through p38 and the motor adaptor Miro/Trak. Cell Rep. 21, 1667–1680 (2017).
Croon, M. et al. FGF21 modulates mitochondrial stress response in cardiomyocytes only under mild mitochondrial dysfunction. Sci. Adv. 8, eabn7105 (2022).
Zorov, D. B., Juhaszova, M. & Sollott, S. J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 94, 909–950 (2014).
Campello, S. et al. Orchestration of lymphocyte chemotaxis by mitochondrial dynamics. J. Exp. Med. 203, 2879–2886 (2006).
Grafstein, B. & Forman, D. S. Intracellular transport in neurons. Physiol. Rev. 60, 1167–1283 (1980).
Eisner, V., Picard, M. & Hajnoczky, G. Mitochondrial dynamics in adaptive and maladaptive cellular stress responses. Nat. Cell Biol. 20, 755–765 (2018).
Spees, J. L., Olson, S. D., Whitney, M. J. & Prockop, D. J. Mitochondrial transfer between cells can rescue aerobic respiration. Proc. Natl Acad. Sci. USA 103, 1283–1288 (2006).
Rustom, A., Saffrich, R., Markovic, I., Walther, P. & Gerdes, H. H. Nanotubular highways for intercellular organelle transport. Science. 303, 1007–1010 (2004).
Liu, D. et al. Intercellular mitochondrial transfer as a means of tissue revitalization. Signal Transduct. Target Ther. 6, 65 (2021).
Liu, Z., Sun, Y., Qi, Z., Cao, L. & Ding, S. Mitochondrial transfer/transplantation: an emerging therapeutic approach for multiple diseases. Cell Biosci. 12, 66 (2022).
Dong, L. F. et al. Horizontal transfer of whole mitochondria restores tumorigenic potential in mitochondrial DNA-deficient cancer cells. eLife 6, e22187 (2017).
McCully, J. D., Levitsky, S., Del Nido, P. J. & Cowan, D. B. Mitochondrial transplantation for therapeutic use. Clin. Transl. Med. 5, 16 (2016).
Hayashida, K. et al. Mitochondrial transplantation therapy for ischemia reperfusion injury: a systematic review of animal and human studies. J. Transl. Med. 19, 214 (2021).
Sardon Puig, L., Valera-Alberni, M., Canto, C. & Pillon, N. J. Circadian rhythms and mitochondria: connecting the dots. Front. Genet. 9, 452 (2018).
Chang, E. M., Chao, C. C., Wang, M. T., Hsu, C. L. & Chen, P. C. PM(2.5) promotes pulmonary fibrosis by mitochondrial dysfunction. Environ. Toxicol. 38, 1905–1913 (2023).
Gioscia-Ryan, R. A. et al. Lifelong voluntary aerobic exercise prevents age- and Western diet- induced vascular dysfunction, mitochondrial oxidative stress and inflammation in mice. J. Physiol. 599, 911–925 (2021).
Acknowledgements
We thank E. L. Mills, D. G. Ryan and H. A. Prag for helpful discussions.
Author information
Authors and Affiliations
Contributions
Both authors contributed equally to the writing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Navdeep Chandel, Zhijian (James) Chen, Luca Scorrano and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Murphy, M.P., O’Neill, L.A.J. A break in mitochondrial endosymbiosis as a basis for inflammatory diseases. Nature 626, 271–279 (2024). https://doi.org/10.1038/s41586-023-06866-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-06866-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.