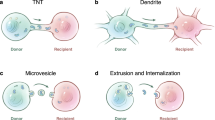
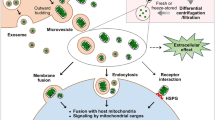
Abstract
Mitochondria are believed to have originated through an ancient endosymbiotic process in which proteobacteria were captured and co-opted for energy production and cellular metabolism. Mitochondria segregate during cell division and differentiation, with vertical inheritance of mitochondria and the mitochondrial DNA genome from parent to daughter cells. However, an emerging body of literature indicates that some cell types export their mitochondria for delivery to developmentally unrelated cell types, a process called intercellular mitochondria transfer. In this Review, we describe the mechanisms by which mitochondria are transferred between cells and discuss how intercellular mitochondria transfer regulates the physiology and function of various organ systems in health and disease. In particular, we discuss the role of mitochondria transfer in regulating cellular metabolism, cancer, the immune system, maintenance of tissue homeostasis, mitochondrial quality control, wound healing and adipose tissue function. We also highlight the potential of targeting intercellular mitochondria transfer as a therapeutic strategy to treat human diseases and augment cellular therapies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
Data sharing is not applicable because no new data were created or analysed in this Review article.
References
Roger, A. J., Muñoz-Gómez, S. A. & Kamikawa, R. The origin and diversification of mitochondria. Curr. Biol. 27, R1177–R1192 (2017).
Monzel, A. S., Enríquez, J. A. & Picard, M. Multifaceted mitochondria: moving mitochondrial science beyond function and dysfunction. Nat. Metab. 5, 546–562 (2023).
Mishra, P. & Chan, D. C. Mitochondrial dynamics and inheritance during cell division, development and disease. Nat. Rev. Mol. Cell Biol. 15, 634–646 (2014).
Spees, J. L., Olson, S. D., Whitney, M. J. & Prockop, D. J. Mitochondrial transfer between cells can rescue aerobic respiration. Proc. Natl Acad. Sci. USA 103, 1283–1288 (2006). This article is one of the first to demonstrate that ρ0 cells can obtain mitochondria from neighbouring cells to rescue aerobic respiration and support energetically demanding processes such as cell division.
Okafo, G., Prevedel, L. & Eugenin, E. Tunneling nanotubes (TNT) mediate long-range gap junctional communication: implications for HIV cell to cell spread. Sci. Rep. 7, 16660 (2017).
Yao, Y. et al. Connexin 43-mediated mitochondrial transfer of iPSC-MSCs alleviates asthma inflammation. Stem Cell Rep. 11, 1120–1135 (2018).
Tishchenko, A. et al. Cx43 and associated cell signaling pathways regulate tunneling nanotubes in breast cancer cells. Cancers 12, 2798 (2020).
Lou, E. et al. Tunneling nanotubes provide a unique conduit for intercellular transfer of cellular contents in human malignant pleural mesothelioma. PLoS ONE 7, e33093 (2012).
Plotnikov, E. Y. et al. Cell-to-cell cross-talk between mesenchymal stem cells and cardiomyocytes in co-culture. J. Cell. Mol. Med. 12, 1622–1631 (2008).
Cselenyák, A., Pankotai, E., Horváth, E. M., Kiss, L. & Lacza, Z. Mesenchymal stem cells rescue cardiomyoblasts from cell death in an in vitro ischemia model via direct cell-to-cell connections. BMC Cell Biol. 11, 29 (2010).
He, K. et al. Long-distance intercellular connectivity between cardiomyocytes and cardiofibroblasts mediated by membrane nanotubes. Cardiovasc. Res. 92, 39–47 (2011).
Vallabhaneni, K. C., Haller, H. & Dumler, I. Vascular smooth muscle cells initiate proliferation of mesenchymal stem cells by mitochondrial transfer via tunneling nanotubes. Stem Cells Dev. 21, 3104–3113 (2012).
Liu, K. et al. Mesenchymal stem cells rescue injured endothelial cells in an in vitro ischemia–reperfusion model via tunneling nanotube like structure-mediated mitochondrial transfer. Microvasc. Res. 92, 10–18 (2014).
Han, H. et al. Bone marrow-derived mesenchymal stem cells rescue injured H9c2 cells via transferring intact mitochondria through tunneling nanotubes in an in vitro simulated ischemia/reperfusion model. Mol. Med. Rep. 13, 1517–1524 (2016).
Yang, H. et al. Biochip-based study of unidirectional mitochondrial transfer from stem cells to myocytes via tunneling nanotubes. Biofabrication 8, 015012 (2016).
Zhang, Y. et al. iPSC-MSCs with high intrinsic MIRO1 and sensitivity to TNF-α yield efficacious mitochondrial transfer to rescue anthracycline-induced cardiomyopathy. Stem Cell Rep. 7, 749–763 (2016).
Shen, J. et al. Mitochondria are transported along microtubules in membrane nanotubes to rescue distressed cardiomyocytes from apoptosis. Cell Death Dis. 9, 81 (2018).
Jackson, M. V. et al. Mitochondrial transfer via tunneling nanotubes is an important mechanism by which mesenchymal stem cells enhance macrophage phagocytosis in the in vitro and in vivo models of ARDS. Stem Cells 34, 2210–2223 (2016).
Jackson, M. V. & Krasnodembskaya, A. D. Analysis of mitochondrial transfer in direct co-cultures of human monocyte-derived macrophages (MDM) and mesenchymal stem cells (MSC). Bio Protoc. 7, e2255 (2017).
Mistry, J. J. et al. ROS-mediated PI3K activation drives mitochondrial transfer from stromal cells to hematopoietic stem cells in response to infection. Proc. Natl Acad. Sci. USA 116, 24610–24619 (2019).
Islam, M. N. et al. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat. Med. 18, 759–765 (2012). This article demonstrates that mitochondria transfer has anti-inflammatory properties in the lung and can protect against acute lung injury in vivo.
Li, X. et al. Mitochondrial transfer of induced pluripotent stem cell-derived mesenchymal stem cells to airway epithelial cells attenuates cigarette smoke-induced damage. Am. J. Respir. Cell Mol. Biol. 51, 455–465 (2014).
Ahmad, T. et al. Miro1 regulates intercellular mitochondrial transport & enhances mesenchymal stem cell rescue efficacy. EMBO J. 33, 994–1010 (2014).
Sinclair, K. A., Yerkovich, S. T., Hopkins, P. M.-A. & Chambers, D. C. Characterization of intercellular communication and mitochondrial donation by mesenchymal stromal cells derived from the human lung. Stem Cell Res. Ther. 7, 91 (2016).
Jiang, D. et al. Mitochondrial transfer of mesenchymal stem cells effectively protects corneal epithelial cells from mitochondrial damage. Cell Death Dis. 7, e2467 (2016).
Pasquier, J. et al. Preferential transfer of mitochondria from endothelial to cancer cells through tunneling nanotubes modulates chemoresistance. J. Transl. Med. 11, 94 (2013).
Osswald, M. et al. Brain tumour cells interconnect to a functional and resistant network. Nature 528, 93–98 (2015).
Desir, S. et al. Tunneling nanotube formation is stimulated by hypoxia in ovarian cancer cells. Oncotarget 7, 43150–43161 (2016).
Lu, J. et al. Tunneling nanotubes promote intercellular mitochondria transfer followed by increased invasiveness in bladder cancer cells. Oncotarget 8, 15539–15552 (2017).
Marlein, C. R. et al. NADPH oxidase-2 derived superoxide drives mitochondrial transfer from bone marrow stromal cells to leukemic blasts. Blood 130, 1649–1660 (2017).
Díaz-Carballo, D. et al. Cytotoxic stress induces transfer of mitochondria-associated human endogenous retroviral RNA and proteins between cancer cells. Oncotarget 8, 95945–95964 (2017).
Ippolito, L. et al. Cancer-associated fibroblasts promote prostate cancer malignancy via metabolic rewiring and mitochondrial transfer. Oncogene 38, 5339–5355 (2019).
Marlein, C. R. et al. CD38-driven mitochondrial trafficking promotes bioenergetic plasticity in multiple myeloma. Cancer Res. 79, 2285–2297 (2019).
Pinto, G. et al. Patient-derived glioblastoma stem cells transfer mitochondria through tunneling nanotubes in tumor organoids. Biochem. J. 478, 21–39 (2021).
Wang, X. & Gerdes, H.-H. Transfer of mitochondria via tunneling nanotubes rescues apoptotic PC12 cells. Cell Death Differ. 22, 1181–1191 (2015).
Babenko, V. A. et al. Improving the post-stroke therapeutic potency of mesenchymal multipotent stromal cells by cocultivation with cortical neurons: the role of crosstalk between cells. Stem Cells Transl. Med. 4, 1011–1020 (2015).
Boukelmoune, N., Chiu, G. S., Kavelaars, A. & Heijnen, C. J. Mitochondrial transfer from mesenchymal stem cells to neural stem cells protects against the neurotoxic effects of cisplatin. Acta Neuropathol. Commun. 6, 139 (2018).
Li, H. et al. Mitochondrial transfer from bone marrow mesenchymal stem cells to motor neurons in spinal cord injury rats via gap junction. Theranostics 9, 2017–2035 (2019).
Nasoni, M. G. et al. Melatonin reshapes the mitochondrial network and promotes intercellular mitochondrial transfer via tunneling nanotubes after ischemic-like injury in hippocampal HT22 cells. J. Pineal Res. 71, e12747 (2021).
Nitzan, K. et al. Mitochondrial transfer ameliorates cognitive deficits, neuronal loss, and gliosis in Alzheimer’s disease mice. J. Alzheimers Dis. 72, 587–604 (2019).
Watson, D. C. et al. GAP43-dependent mitochondria transfer from astrocytes enhances glioblastoma tumorigenicity. Nat. Cancer 4, 648–664 (2023).
Crewe, C. et al. Extracellular vesicle-based interorgan transport of mitochondria from energetically stressed adipocytes. Cell Metab. 33, 1853–1868.e11 (2021). This article shows that metabolically stressed adipocytes release mitochondria in extracellular vesicles into circulation for delivery to other organs, such as the heart, where they metabolically precondition the heart to surmount ischaemia–reperfusion injury.
Rosina, M. et al. Ejection of damaged mitochondria and their removal by macrophages ensure efficient thermogenesis in brown adipose tissue. Cell Metab. 34, 533–548.e12 (2022).
Suh, J. et al. Mitochondrial fragmentation and donut formation enhance mitochondrial secretion to promote osteogenesis. Cell Metab. 35, 345–360.e7 (2023).
Nicolás-Ávila, J. A. et al. A network of macrophages supports mitochondrial homeostasis in the heart. Cell 183, 94–109.e23 (2020). This article reports that metabolically stressed adipocytes eject exophers containing damaged mitochondria and deliver them to tissue-resident macrophages for elimination, thereby supporting maintenance of mitochondria quality control in the donor cardiomyocytes.
Liang, W. et al. Mitochondria are secreted in extracellular vesicles when lysosomal function is impaired. Nat. Commun. 14, 5031 (2023).
Bucci, C., Thomsen, P., Nicoziani, P., McCarthy, J. & van Deurs, B. Rab7: a key to lysosome biogenesis. Mol. Biol. Cell 11, 467–480 (2000).
Boudreau, L. H. et al. Platelets release mitochondria serving as substrate for bactericidal group IIA-secreted phospholipase A2 to promote inflammation. Blood 124, 2173–2183 (2014).
Hayakawa, K. et al. Transfer of mitochondria from astrocytes to neurons after stroke. Nature 535, 551–555 (2016). This article reveals that astrocytes transfer mitochondria to neurons to limit ischaemic stroke pathology, indicating that intercellular mitochondria transfer can be elicited in disease states to mitigate tissue damage.
van der Vlist, M. et al. Macrophages transfer mitochondria to sensory neurons to resolve inflammatory pain. Neuron 110, 613–626.e9 (2022).
Peruzzotti-Jametti, L. et al. Neural stem cells traffic functional mitochondria via extracellular vesicles. PLoS Biol. 19, e3001166 (2021).
Dache, Z. A. A. et al. Blood contains circulating cell-free respiratory competent mitochondria. FASEB J. 34, 3616–3630 (2020).
Borcherding, N. et al. Dietary lipids inhibit mitochondria transfer to macrophages to divert adipocyte-derived mitochondria into blood. Cell Metab. 34, 1499–1513.e8 (2022). This article shows that LCFAs determine whether adipocyte-derived mitochondria are transferred locally to macrophages or diverted into the circulation for delivery to distant organs, and it shows that in vivo administration of purified mitochondria can rescue cell-intrinsic defects in aerobic respiration.
Stephens, O. R. et al. Characterization and origins of cell-free mitochondria in healthy murine and human blood. Mitochondrion 54, 102–112 (2020).
Stier, A. Human blood contains circulating cell-free mitochondria, but are they really functional? Am. J. Physiol. Endocrinol. Metab. 320, E859–E863 (2021).
Joshi, A. U. et al. Fragmented mitochondria released from microglia trigger A1 astrocytic response and propagate inflammatory neurodegeneration. Nat. Neurosci. 22, 1635–1648 (2019).
Levoux, J. et al. Platelets facilitate the wound-healing capability of mesenchymal stem cells by mitochondrial transfer and metabolic reprogramming. Cell Metab. 33, 283–299.e9 (2021). This article reports that platelet-derived mitochondria are captured by mesenchymal stem cells to promote wound healing of skin, implicating intercellular mitochondria transfer in the repair and maintenance of barrier surfaces.
Brestoff, J. R. et al. Intercellular mitochondria transfer to macrophages regulates white adipose tissue homeostasis and is impaired in obesity. Cell Metab. 33, 270–282.e8 (2021). This article demonstrates that adipocytes transfer mitochondria to macrophages in a heparan sulfate-dependent process that is impaired in obesity and links disruption in mitochondria transfer to macrophages with increased susceptibility to obesity.
Mulloy, B. & Forster, M. J. Conformation and dynamics of heparin and heparan sulfate. Glycobiology 10, 1147–1156 (2000).
Cowan, D. B. et al. Transit and integration of extracellular mitochondria in human heart cells. Sci. Rep. 7, 17450 (2017).
Kim, M. J., Hwang, J. W., Yun, C.-K., Lee, Y. & Choi, Y.-S. Delivery of exogenous mitochondria via centrifugation enhances cellular metabolic function. Sci. Rep. 8, 3330 (2018).
Caicedo, A. et al. MitoCeption as a new tool to assess the effects of mesenchymal stem/stromal cell mitochondria on cancer cell metabolism and function. Sci. Rep. 5, 9073 (2015).
McCully, J. D. et al. Injection of isolated mitochondria during early reperfusion for cardioprotection. Am. J. Physiol. Heart Circ. Physiol. 296, H94–H105 (2009).
Masuzawa, A. et al. Transplantation of autologously derived mitochondria protects the heart from ischemia–reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 304, H966–H982 (2013).
Emani, S. M., Piekarski, B. L., Harrild, D., Nido, P. J. D. & McCully, J. D. Autologous mitochondrial transplantation for dysfunction after ischemia–reperfusion injury. J. Thorac. Cardiovasc. Surg. 154, 286–289 (2017).
Norat, P. et al. Intraarterial transplantation of mitochondria after ischemic stroke reduces cerebral infarction. Stroke Vasc. Interv. Neurol. 3, e000644 (2023).
Chou, S. H.-Y. et al. Extracellular mitochondria in cerebrospinal fluid and neurological recovery after subarachnoid hemorrhage. Stroke 48, 2231–2237 (2017).
Tan, A. S. et al. Mitochondrial genome acquisition restores respiratory function and tumorigenic potential of cancer cells without mitochondrial DNA. Cell Metab. 21, 81–94 (2015).
Dong, L.-F. et al. Horizontal transfer of whole mitochondria restores tumorigenic potential in mitochondrial DNA-deficient cancer cells. eLife 6, e22187 (2017).
Kidwell, C. U. et al. Transferred mitochondria accumulate reactive oxygen species, promoting proliferation. eLife 12, e85494 (2023).
Salaud, C. et al. Mitochondria transfer from tumor-activated stromal cells (TASC) to primary glioblastoma cells. Biochem. Biophys. Res. Commun. 533, 139–147 (2020).
Rabas, N. et al. PINK1 drives production of mtDNA-containing extracellular vesicles to promote invasiveness. J. Cell Biol. 220, e202006049 (2021).
Saha, T. et al. Intercellular nanotubes mediate mitochondrial trafficking between cancer and immune cells. Nat. Nanotechnol. 17, 98–106 (2022). This article demonstrates that cancer cells deplete tumour-infiltrating lymphocytes of mitochondria to simultaneously support their own energetic demands and impair antitumour immunity.
Chang, J.-C. et al. Mitochondrial transplantation regulates antitumour activity, chemoresistance and mitochondrial dynamics in breast cancer. J. Exp. Clin. Cancer Res. 38, 30 (2019).
Moschoi, R. et al. Protective mitochondrial transfer from bone marrow stromal cells to acute myeloid leukemic cells during chemotherapy. Blood 128, 253–264 (2016).
Hutto, R. A. et al. Cone photoreceptors transfer damaged mitochondria to Müller glia. Cell Rep. https://doi.org/10.1016/j.celrep.2023.112115 (2023).
Hayakawa, K. et al. Protective effects of endothelial progenitor cell-derived extracellular mitochondria in brain endothelium. Stem Cells 36, 1404–1410 (2018).
Liang, X. et al. Direct administration of mesenchymal stem cell-derived mitochondria improves cardiac function after infarction via ameliorating endothelial senescence. Bioeng. Transl. Med. 8, e10365 (2022).
Weiß, E. & Kretschmer, D. Formyl-peptide receptors in infection, inflammation, and cancer. Trends Immunol. 39, 815–829 (2018).
Wu, G. et al. Extracellular mitochondrial DNA promote NLRP3 inflammasome activation and induce acute lung injury through TLR9 and NF-κB. J. Thorac. Dis. https://doi.org/10.21037/jtd.2019.10.26 (2019).
Scozzi, D. et al. Mitochondrial damage-associated molecular patterns released by lung transplants are associated with primary graft dysfunction. Am. J. Transplant. 19, 1464–1477 (2019).
Pollara, J., Edwards, R. W., Lin, L., Bendersky, V. A. & Brennan, T. V. Circulating mitochondria in deceased organ donors are associated with immune activation and early allograft dysfunction. JCI Insight https://doi.org/10.1172/jci.insight.121622 (2018).
Luz-Crawford, P. et al. Mesenchymal stem cell repression of Th17 cells is triggered by mitochondrial transfer. Stem Cell Res. Ther. 10, 232 (2019).
Court, A. C. et al. Mitochondrial transfer from MSCs to T cells induces Treg differentiation and restricts inflammatory response. EMBO Rep. 21, e48052 (2020).
Wu, B. et al. Mitochondrial aspartate regulates TNF biogenesis and autoimmune tissue inflammation. Nat. Immunol. 22, 1551–1562 (2021).
Giwa, R. & Brestoff, J. R. Mitochondria transfer to CD4+ T cells may alleviate rheumatoid arthritis by suppressing pro-inflammatory cytokine production. Immunometabolism https://doi.org/10.20900/immunometab20220009 (2022).
Huang, Y. et al. TP53/p53 facilitates stress-induced exosome and protein secretion by adipocytes. Diabetes https://doi.org/10.2337/db22-1027 (2023).
Gao, J. et al. Endoplasmic reticulum mediates mitochondrial transfer within the osteocyte dendritic network. Sci. Adv. 5, eaaw7215 (2019).
Herbert, M. & Turnbull, D. Progress in mitochondrial replacement therapies. Nat. Rev. Mol. Cell Biol. 19, 71–72 (2018).
Barritt, J. A., Brenner, C. A., Malter, H. E. & Cohen, J. Mitochondria in human offspring derived from ooplasmic transplantation. Hum. Reprod. 16, 513–516 (2001).
Jacoby, E. et al. Mitochondrial augmentation of CD34+ cells from healthy donors and patients with mitochondrial DNA disorders confers functional benefit. npj Regen. Med. 6, 58 (2021).
Jacoby, E. et al. Mitochondrial augmentation of hematopoietic stem cells in children with single large-scale mitochondrial DNA deletion syndromes. Sci. Transl. Med. 14, eabo3724 (2022).
Ikeda, G. et al. Mitochondria-rich extracellular vesicles from autologous stem cell-derived cardiomyocytes restore energetics of ischemic myocardium. J. Am. Coll. Cardiol. 77, 1073–1088 (2021).
Nakamura, Y., Park, J.-H. & Hayakawa, K. Therapeutic use of extracellular mitochondria in CNS injury and disease. Exp. Neurol. 324, 113114 (2020).
Huang, P.-J. et al. Transferring xenogenic mitochondria provides neural protection against ischemic stress in ischemic rat brains. Cell Transplant. 25, 913–927 (2016).
Acknowledgements
J.R.B. is supported by the NIH Director’s Early Independence Award (DP5 OD028125) and the Burroughs Wellcome Fund Career Award for Medical Scientists (CAMS 1019648).
Author information
Authors and Affiliations
Contributions
N.B. and J.R.B. wrote and approved the manuscript. J.R.B. conceived of the work and supervised its development.
Corresponding author
Ethics declarations
Competing interests
J.R.B. has pending patent applications related to mitochondria transplantation for the treatment of mitochondrial disorders and lipid metabolism; immunoassays for serum-free light chains; and an immunotherapy for atopic dermatitis. He is a member of the Scientific Advisory Board for LUCA Science, Inc., has consulted for DeciBio and Flagship Pioneering within the past 12 months, and receives royalties from Springer Nature Group. N.B. is a consultant for Santa Ana Bio and an advisor for Omniscope.
Peer review
Peer review information
Nature thanks Anne-Marie Rodriguez, Gerald Shadel and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Borcherding, N., Brestoff, J.R. The power and potential of mitochondria transfer. Nature 623, 283–291 (2023). https://doi.org/10.1038/s41586-023-06537-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-06537-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.