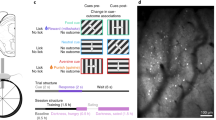
Abstract
Many theories of offline memory consolidation posit that the pattern of neurons activated during a salient sensory experience will be faithfully reactivated, thereby stabilizing the pattern1,2. However, sensory-evoked patterns are not stable but, instead, drift across repeated experiences3,4,5,6. Here, to investigate the relationship between reactivations and the drift of sensory representations, we imaged the calcium activity of thousands of excitatory neurons in the mouse lateral visual cortex. During the minute after a visual stimulus, we observed transient, stimulus-specific reactivations, often coupled with hippocampal sharp-wave ripples. Stimulus-specific reactivations were abolished by local cortical silencing during the preceding stimulus. Reactivations early in a session systematically differed from the pattern evoked by the previous stimulus—they were more similar to future stimulus response patterns, thereby predicting both within-day and across-day representational drift. In particular, neurons that participated proportionally more or less in early stimulus reactivations than in stimulus response patterns gradually increased or decreased their future stimulus responses, respectively. Indeed, we could accurately predict future changes in stimulus responses and the separation of responses to distinct stimuli using only the rate and content of reactivations. Thus, reactivations may contribute to a gradual drift and separation in sensory cortical response patterns, thereby enhancing sensory discrimination7.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Processed imaging and behavioural data are available online (https://research.bidmc.harvard.edu/datashare/DataShareInfo.ASP?Submit=Display&ID=11). Raw imaging and behavioural data are available on request.
Code availability
Code is available at GitHub (https://github.com/nguyenr95/reactivation).
References
Foster, D. J. Replay comes of age. Annu. Rev. Neurosci. 40, 581–602 (2017).
Tambini, A. & Davachi, L. Awake reactivation of prior experiences consolidates memories and biases cognition. Trends Cogn. Sci. 23, 876–890 (2019).
Failor, S. W., Carandini, M. & Harris, K. D. Visuomotor association orthogonalizes visual cortical population codes. Preprint at bioRxiv https://doi.org/10.1101/2021.05.23.445338 (2022).
Schoonover, C. E. et al. Representational drift in primary olfactory cortex. Nature 594, 541–546 (2021).
Marks, T. D. & Goard, M. J. Stimulus-dependent representational drift in primary visual cortex. Nat. Commun. 12, 5169 (2021).
Deitch, D., Rubin, A. & Ziv, Y. Representational drift in the mouse visual cortex. Curr. Biol. 31, 4327–4339 (2021).
Clifford, C. W. et al. Orthogonal adaptation improves orientation discrimination. Vision Res. 41, 151–159 (2001).
Karlsson, M. P. & Frank, L. M. Awake replay of remote experiences in the hippocampus. Nat. Neurosci. 12, 913–918 (2009).
Lee, A. K. & Wilson, M. A. Memory of sequential experience in the hippocampus during slow wave sleep. Neuron 36, 1183–1194 (2002).
Nadasdy, Z. et al. Replay and time compression of recurring spike sequences in the hippocampus. J. Neurosci. 19, 9497–9507 (1999).
Tang, W. et al. Hippocampal-prefrontal reactivation during learning is stronger in awake compared with sleep states. J. Neurosci. 37, 11789–11805 (2017).
Carrillo-Reid, L. et al. Imprinting and recalling cortical ensembles. Science 353, 691–694 (2016).
Ji, D. & Wilson, M. A. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat. Neurosci. 10, 100–107 (2007).
O’Neill, J. et al. Superficial layers of the medial entorhinal cortex replay independently of the hippocampus. Science 355, 184–188 (2017).
Reitich-Stolero, T. & Paz, R. Affective memory rehearsal with temporal sequences in amygdala neurons. Nat. Neurosci. 22, 2050–2059 (2019).
Rothschild, G., Eban, E. & Frank, L. M. A cortical-hippocampal-cortical loop of information processing during memory consolidation. Nat. Neurosci. 20, 251–259 (2017).
Shin, J. D., Tang, W. & Jadhav, S. P. Dynamics of awake hippocampal-prefrontal replay for spatial learning and memory-guided decision making. Neuron 104, 1110–1125 (2019).
Sugden, A. U. et al. Cortical reactivations of recent sensory experiences predict bidirectional network changes during learning. Nat. Neurosci. 23, 981–991 (2020).
Euston, D. R., Tatsuno, M. & McNaughton, B. L. Fast-forward playback of recent memory sequences in prefrontal cortex during sleep. Science 318, 1147–1150 (2007).
Khodagholy, D., Gelinas, J. N. & Buzsaki, G. Learning-enhanced coupling between ripple oscillations in association cortices and hippocampus. Science 358, 369–372 (2017).
Lines, J. & Yuste, R. Visually evoked neuronal ensembles reactivate during sleep. Preprint at bioRxiv https://doi.org/10.1101/2023.04.26.538480 (2023).
Chang, H. et al. Cortical reactivation of non-spatial and spatial memory representations coordinate with hippocampus to form a memory dialogue. Preprint at bioRxiv https://doi.org/10.1101/2022.12.16.520658 (2022).
Eagleman, S. L. & Dragoi, V. Image sequence reactivation in awake V4 networks. Proc. Natl Acad. Sci. USA 109, 19450–19455 (2012).
Genzel, L. et al. A consensus statement: defining terms for reactivation analysis. Philos. Trans. R. Soc. Lond. B 375, 20200001 (2020).
Swanson, R. A. et al. Variable specificity of memory trace reactivation during hippocampal sharp wave ripples. Curr. Opin. Behav. Sci. 32, 126–135 (2020).
Gupta, A. S. et al. Hippocampal replay is not a simple function of experience. Neuron 65, 695–705 (2010).
Terada, S. et al. Adaptive stimulus selection for consolidation in the hippocampus. Nature 601, 240–244 (2022).
Stringer, C. et al. Spontaneous behaviors drive multidimensional, brainwide activity. Science 364, 255 (2019).
Cooke, S. F. et al. Visual recognition memory, manifested as long-term habituation, requires synaptic plasticity in V1. Nat. Neurosci. 18, 262–271 (2015).
Frenkel, M. Y. et al. Instructive effect of visual experience in mouse visual cortex. Neuron 51, 339–349 (2006).
Dana, H. et al. High-performance calcium sensors for imaging activity in neuronal populations and microcompartments. Nat. Methods 16, 649–657 (2019).
Gorski, J. A. et al. Cortical excitatory neurons and glia, but not GABAergic neurons, are produced in the Emx1-expressing lineage. J. Neurosci. 22, 6309–6314 (2002).
Ramesh, R. N. et al. Intermingled ensembles in visual association cortex encode stimulus identity or predicted outcome. Neuron 100, 900–915 (2018).
Zhuang, J. et al. An extended retinotopic map of mouse cortex. eLife. 6, e18372 (2017).
Bradley, M. M. et al. The pupil as a measure of emotional arousal and autonomic activation. Psychophysiology 45, 602–607 (2008).
Jeong, H. et al. Sensory cortical ensembles exhibit differential coupling to ripples in distinct hippocampal subregions. Curr. Biol. https://doi.org/10.1016/j.cub.2023.10.073 (2023).
Berners-Lee, A. et al. Hippocampal replays appear after a single experience and incorporate greater detail with more experience. Neuron 110, 1829–1842 e5 (2022).
Gillespie, A. K. et al. Hippocampal replay reflects specific past experiences rather than a plan for subsequent choice. Neuron 109, 3149–3163 (2021).
Singer, A. C. & Frank, L. M. Rewarded outcomes enhance reactivation of experience in the hippocampus. Neuron 64, 910–921 (2009).
Zutshi, I. & Buzsaki, G. Hippocampal sharp-wave ripples and their spike assembly content are regulated by the medial entorhinal cortex. Curr. Biol. 33, 3648–3659 (2023).
Vormstein-Schneider, D. et al. Viral manipulation of functionally distinct interneurons in mice, non-human primates and humans. Nat. Neurosci. 23, 1629–1636 (2020).
Schmid, C. et al. Passive exposure to task-relevant stimuli enhances categorization learning. eLife 12, RP88406 (2023).
McGuire, K. L. et al. Visual association cortex links cues with conjunctions of reward and locomotor contexts. Curr. Biol. 32, 1563–1576 (2022).
Slomowitz, E. et al. Interplay between population firing stability and single neuron dynamics in hippocampal networks. eLife 4, e04378 (2015).
Hengen, K. B. et al. Firing rate homeostasis in visual cortex of freely behaving rodents. Neuron 80, 335–342 (2013).
Roux, L. et al. Sharp wave ripples during learning stabilize the hippocampal spatial map. Nat. Neurosci. 20, 845–853 (2017).
Grosmark, A. D. et al. Reactivation predicts the consolidation of unbiased long-term cognitive maps. Nat. Neurosci. 24, 1574–1585 (2021).
van de Ven, G. M. et al. Hippocampal offline reactivation consolidates recently formed cell assembly patterns during sharp wave-ripples. Neuron 92, 968–974 (2016).
Jun, J. J. et al. Fully integrated silicon probes for high-density recording of neural activity. Nature 551, 232–236 (2017).
Ego-Stengel, V. & Wilson, M. A. Disruption of ripple-associated hippocampal activity during rest impairs spatial learning in the rat. Hippocampus 20, 1–10 (2010).
Jadhav, S. P. et al. Awake hippocampal sharp-wave ripples support spatial memory. Science 336, 1454–1458 (2012).
Girardeau, G. et al. Selective suppression of hippocampal ripples impairs spatial memory. Nat. Neurosci. 12, 1222–1223 (2009).
Fauth, M. J. & van Rossum, M. C. Self-organized reactivation maintains and reinforces memories despite synaptic turnover. eLife 8, e43717 (2019).
Mau, W., Hasselmo, M. E. and Cai, D. J. The brain in motion: how ensemble fluidity drives memory-updating and flexibility. eLife 9, e63550 (2020).
Hanert, A. et al. Sleep in humans stabilizes pattern separation performance. J. Neurosci. 37, 12238–12246 (2017).
Miller, J. E. et al. Visual stimuli recruit intrinsically generated cortical ensembles. Proc. Natl Acad. Sci. USA 111, E4053–E4061 (2014).
Vaz, A. P. et al. Backbone spiking sequence as a basis for preplay, replay, and default states in human cortex. Nat. Commun. 14, 4723 (2023).
Karlsson, M. P. & Frank, L. M. Network dynamics underlying the formation of sparse, informative representations in the hippocampus. J. Neurosci. 28, 14271–14281 (2008).
Rolls, E. T. The mechanisms for pattern completion and pattern separation in the hippocampus. Front. Syst. Neurosci. 7, 74 (2013).
McClelland, J. L., McNaughton, B. L. & O’Reilly, R. C. Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol. Rev. 102, 419–457 (1995).
Liang, L. et al. Retinal inputs to the thalamus are selectively gated by arousal. Curr. Biol. 30, 3923–3934 (2020).
Goldey, G. J. et al. Removable cranial windows for long-term imaging in awake mice. Nat. Protoc. 9, 2515–2538 (2014).
Wang, Q. & Burkhalter, A. Area map of mouse visual cortex. J. Comp. Neurol. 502, 339–357 (2007).
Pachitariu, M. et al. Suite2p: beyond 10,000 neurons with standard two-photon microscopy. Preprint at bioRxiv https://doi.org/10.1101/061507 (2017).
Friedrich, J., Zhou, P. & Paninski, L. Fast online deconvolution of calcium imaging data. PLoS Comput Biol. 13, e1005423 (2017).
Syeda, A. et al. Facemap: a framework for modeling neural activity based on orofacial tracking. Preprint at bioRxiv https://doi.org/10.1101/2022.11.03.515121 (2022).
Acknowledgements
We thank C. Harvey, B. McNaughton, S. Zhang, R. Essner, A. Lowet, D. Tingley, A. Sugden, J. Zaremba, M. Nguyen and the members of the Andermann laboratory for feedback; A. Sambangi for help validating AAV-S5E2-Chrimson; and K. Lensjø for advice on AAV-PHP.eb-jGCaMP7s. This project was supported by a National Defense Science and Engineering Fellowship and a Howard Hughes Medical Institute Gilliam Fellowship (to N.D.N.), NIH F32 DK112589 and Davis Family Foundation awards (to A.L.), and NIH DP2 DK105570, R01 MH12343, DP1 AT010971, a McKnight Scholar Award and a Harvard Brain Science Initiative Bipolar Disorder Seed Grant, supported by K. and L. Dauten (to M.L.A.). Icons in Figs. 1a,b,f and 2d were created using BioRender.
Author information
Authors and Affiliations
Contributions
N.D.N. and M.L.A. conceived the project, designed the experiments and analyses, and wrote the manuscript. N.D.N. performed experiments and analysed the data. A.L. designed and helped with optogenetic silencing studies. O.A. and A.Y.-E.A. performed and analysed the hippocampal ripple experiments. J.F. helped with surgical procedures. R.H. and B.L.S. developed the cross-day tracking analysis. J.V., J.M. and J.D. provided the AAV-S5E2-Chrimson-tdTomato virus.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Pieter Goltstein and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Classifying stimulus-specific reactivations.
a, Trial-averaged, deconvolved peri-stimulus Ca2+ activity of example neurons driven by S1, S2, or both (“S1 and S2 neurons”). S1- and S2-driven neurons exhibited highly selective responses to their preferred stimulus. b, Quantification of neuron count for: all neurons, S1 and S2 neurons, S1 only neurons, and S2 only neurons (average across all trials, and separately for early and for late trials, n = 8 mice). c, Distribution of selectivity index values (see Methods) of stimulus driven neurons (n = 8 mice). d, Brief summary of method for classifying reactivations (for additional details, see main text and Methods). Left (Step 1): the classifier should identify transient synchronous reactivations that we assume should last at least several hundred milliseconds1,9,13,16,18, and thus we estimate population activity patterns using the rolling maximum activity of each cell across ~380 ms. We then remove slow changes in ongoing Ca2+ activity by using 3 difference-of-Gaussian filters to high-pass filter activity changes at time scales of 1.5, 6, and 25 s. Middle (Step 2): we define S1 or S2 stimulus reactivations during the inter-trial interval (in which the mouse passively views a mean-luminance blank screen) as epochs of synchronous activity lasting hundreds of milliseconds across neurons driven by stimulus S1 or S2, respectively. To focus on synchronous events, we use a binary prior such that we only classify reactivation pattern content during epochs in which the ongoing activity trace averaged across the top stimulus-driven neurons exceeds 5 standard deviations above the mean. Right (Step 3): we then apply multinomial logistic regression to epochs specified by this temporal prior. We train the classifier on time points that occur during all S1 trials, all S2 trials, and all time points during inter-trial intervals and during the baseline period that do not exhibit synchronous activity of stimulus-driven neurons (temporal prior = 0). We then apply the classifier to all time points with synchronous activity of stimulus-driven neurons during inter-trial intervals and during the baseline period prior to any stimulus presentations (temporal prior = 1). This results in matching probability estimates that the pattern at each time point matches the S1-evoked response pattern, the S2-evoked response pattern (i.e. S1 or S2 reactivation probabilities), or ‘other’ patterns, with the sum of these three probabilities equalling 1 for each time point. e, Reactivation duration during the baseline period before any stimulus presentations vs. during the inter-trial intervals between stimulus presentations (n = 8 mice, two-tailed paired t-test, P = 0.025). f, Left: distribution of reactivation probabilities of the classifier trained using the actual data and trained using data after shuffling using one of two different methods. The first shuffle method defines the temporal prior using an equal number of randomly selected neurons instead of only stimulus-driven neurons, and the second method randomly shuffles the identity of stimulus-driven neurons (n = 8 mice; one-way ANOVA, Holm-Bonferroni corrected, all data points below the significance line indicate classifier probabilities that differ significantly from both shuffled versions, P < .05). Right: fold change in density of each reactivation probability using the actual data as compared to each shuffle (n = 8 mice). We defined reactivation events as those with a peak probability greater than 0.75, as they were greater than three times more common than reactivations detected in shuffled data. g, Reactivation rate during times of synchronous stimulus activity during the ITI vs. all other ITI times with non-synchronous stimulus activity (n = 8 mice, two-tailed paired t-test, P = 3.6 x 10−7). Classifier probability during the ITI was low outside of moments of synchronous activation of stimulus-driven neurons. In this case, classification of reactivations was performed without removing slow changes in ongoing Ca2+ activity using the 3 difference-of-Gaussian filters to preserve all activity during the ITI. h, We confirmed the similarity of stimulus reactivations to stimulus-evoked response patterns by grouping neurons based on their mean response magnitude during stimulus presentations. As expected, the neurons most strongly driven by S1 or S2 were selectively active during S1 or S2 reactivations, respectively. Left: mean S1-evoked activity (green) or S2-evoked activity (red) for the top 5% and bottom 95% of S1- or S2-driven neurons and for other neurons lacking stimulus-evoked Ca2+ activity (n = 8 mice, two-tailed paired t-test, Holm-Bonferroni corrected, from left to right: P = 0.0012, P = 1.9 × 10−4, P = 0.0013, P = 0.0012, P = 0.42). Middle: same as left but for mean Ca2+ activity during reactivation events (n = 8 mice, two-tailed paired t-test, Holm-Bonferroni corrected, from left to right: P = 0.0017, P = 6.0 x 10−4, P = 0.50, P = 0.86, P = 0.55). Right: baseline Ca2+ activity (in the 0.5 h prior to any stimulus presentations) for the top 5% and bottom 95% of S1- or S2-driven neurons and for other neurons lacking stimulus-evoked Ca2+ activity (n = 8 mice). i, Fraction of neurons that remained in the top 5% of driven neurons during both early trials and late trials (n = 8 mice). Data are mean ± SEM. n.s.: not significant; * P < .05; ** P < .01; *** P < .001; **** P < .0001.
Extended Data Fig. 2 Characterizing stimulus reactivations.
a, Mean hippocampal ripple-band power surrounding the onset of classified reactivations (derived from the cortical imaging data) across each session for all mice (n = 14 sessions from 5 mice that differed from the mice used for any other analyses). SD: standard deviations above the mean. b, Brain motion plotted surrounding the onset of classified reactivations (n = 8 mice, two-tailed paired t-test, P = 0.19). c, Phase correlation to the reference frame, plotted surrounding the onset of classified reactivations (n = 8 mice, two-tailed paired t-test, P = 0.011). The phase correlation measures how well each individual frame correlates with the reference frame used for motion correction. d, Peak-normalized pupil movement (absolute change in movement) plotted surrounding the onset of classified reactivation events (n = 8 mice, two-tailed paired t-test, P = 0.47). e, Comparison of mean stimulus-evoked activity (left) or stimulus reactivation activity (right) between neurons located in upper layer 2/3 (~ 156 μm from the brain surface) vs. lower layer 2/3 (~ 266 μm from the brain surface) of lateral visual cortex (n = 8 mice, two-tailed unpaired t-test, stimulus: P = 0.89, reactivation: P = 0.13). f, Change in mean location (centroid, estimated using each stimulus-driven neuron’s activity during reactivations) of stimulus reactivations across the session along the anterior-posterior (left) and lateral-medial axes (right, n = 8 mice, two-tailed paired t-test, Holm-Bonferroni corrected, left: S1: P = 0.035, S2: P = 0.035, right: S1: P = 0.060, S2: P = 0.065). g, Raster plot of ongoing deconvolved Ca2+ activity of the top stimulus-driven neurons during and following an example S1 stimulus presentation (green square) and an example S2 stimulus presentation (red square), using all neurons or using a random 10% of neurons (see lower raster). Classification of stimulus reactivations using a random 10% of neurons results in several false positive (blue arrows) and false negative (magenta arrow) classification errors when compared to using all neurons. Inset at right: expanded view of data from green rectangle, illustrating a false-positive classification using a random 10% of neurons. h, Percent of false negative (left) or false positive (right) classifications of reactivations relative to reactivations classified using all neurons, plotted as a function of the percent of randomly selected neurons used in the classifier (n = 8 mice, permutation test, Holm-Bonferroni corrected, P < .05 for all tests). i, Same as h but selecting neurons randomly from the same subregion of the field of view such that they are all close in distance (see Methods, n = 8 mice, permutation test, Holm-Bonferroni corrected, P < .05 for all tests). Data are mean ± SEM. n.s.: not significant; * P < .05.
Extended Data Fig. 3 Reactivation rate and bias effects are consistent across sessions and correlate with stimulus novelty and pupil-indexed arousal.
a, Left: reactivation rates (sum of probabilities of S1 or S2 reactivations) across each session, including the 0.5-hour baseline period prior to any stimulus presentations for all daily sessions (n = 5 mice, 48 sessions total). Right: reactivation rate during the inter-trial interval (n = 5 mice, 48 sessions total) for all daily sessions. b, Left: bias index of reactivation content (positive values indicate bias towards the most recent stimulus, n = 5 mice, 48 sessions total) for all daily sessions. Right: bias throughout the inter-trial interval (n = 5 mice, 48 sessions total) for all daily sessions. c, Reactivation content bias during stimulus presentations across the session using all neurons vs. a random 10% of neurons (n = 8 mice, permutation test between mean of traces, P = 0.0016). d, Stimulus reactivation rates when the stimulus on the preceding trial was different vs. when it was the same as on the current trial (n = 8 mice, one-tailed t-test vs. 0, P = 9.8 x 10−4). e, Correlation between pupil area during stimulus presentation and stimulus reactivation rate during the subsequent ITI (n = 8 mice, one-tailed t-test vs. 0, P = 0.026). f, Correlation between stimulus activity during stimulus presentation and stimulus reactivation rate during the subsequent ITI (n = 8 mice, one-tailed t-test vs. 0, P = 0.033). Data are mean ± SEM. n.s.: not significant; * P < .05; ** P < .01; *** P < .001.
Extended Data Fig. 4 Physical correlates of arousal remain constant throughout the session.
a, Left: peak-normalized pupil area during stimulus presentations across trials (n = 8 mice, two-tailed paired t-test, P = 0.056). Right: brain motion during stimulus presentations across trials (n = 8 mice, two-tailed paired t-test, P = 0.48). Coloured lines: individual mice. Black line: mean across mice. b, Left: Ca2+ activity during the baseline period before any stimulus presentation (dark shaded region) and during inter-trial intervals between stimulus presentations (n = 8 mice, two-tailed paired t-test, P = 0.016, two-tailed linear least-squares regression, P = 0.018, Holm-Bonferroni corrected). Right: Ca2+ activity during stimulus presentation and throughout the inter-trial interval (n = 8 mice, two-tailed linear least-squares regression, P = 1.2 x 10−19). Dark shaded region: stimulus presentation. Light shaded region: excluded portion of inter-trial interval. Coloured lines: individual mice. Black line: mean across mice. c, Top: peak-normalized pupil area during the baseline period before any stimulus presentation and during inter-trial intervals between stimulus presentations (n = 8 mice, two-tailed paired t-test, P = 0.019, two-tailed linear least-squares regression, P = 0.092, Holm-Bonferroni corrected). Bottom: peak-normalized pupil area during stimulus presentation and throughout the inter-trial interval (n = 8 mice, two-tailed linear least-squares regression, P = 1.7 × 10−6). Dark shaded region: stimulus presentation. Light shaded region: excluded portion of inter-trial interval. Coloured lines: individual mice. Black line: mean across mice. d, Left: example image from a recording of the mouse’s face during imaging. Each coloured dot denotes a keypoint on the face that was tracked across each session. Right: example traces of 8 tracked keypoints on the nose, whiskers, and mouth (nose top, nose tip, nose bottom, whiskers I-III, mouth, and lower lip). Traces are in units of absolute movement. e, Absolute movement of 8 tracked keypoints (nose top, nose tip, nose bottom, whiskers I-III, mouth, and lower lip) during the baseline period before any stimulus presentation and during 2.5 h of stimulus presentations (n = 3 mice, two-tailed linear least-squares regression, nose top: P = 0.39, nose tip: P = 0.54, nose bottom: P = 0.51, whisker I: P = 0.21, whisker II: P = 0.18, whisker III: P = 0.40, mouth: P = 0.19, lower lip: P = 0.70). Data are mean ± SEM. n.s.: not significant; * P < .05; **** P < .0001.
Extended Data Fig. 5 Characterizing the effects of peri-stimulus inhibition.
a, Coronal sections of visual cortex displaying virally-mediated expression of Cre-dependent jGCaMP7s in glutamatergic neurons (green, in Emx1-Cre mice) and Chrimson in parvalbumin interneurons (red, S5E2 enhancer) in 3 mice. Local injections ensured targeted expression of Chrimson throughout lateral visual cortical areas in all 3 mice. b, Left: stimulus-evoked Ca2+ activity during control vs. stimulus-inhibition trials (n = 3 mice, two-tailed paired t-test, P = 0.0056). Right: percent reduction in Ca2+ activity on stimulus-inhibition trials compared to control trials (n = 3 mice, one-sample t-test vs. 0, P = 0.0022). For stimulus-inhibition trials, we pulsed 10 mW of red light for 4 ms at 16 Hz from 1 s before stimulus onset to 1 s after stimulus offset. c, Peak-normalized pupil area during stimulus presentation and during the inter-trial interval for control vs. stimulus-inhibition trials (n = 3 mice, two-tailed paired t-test between mean of traces during the stimulus period plus the period immediately following the stimulus, P = 0.75, or during the specified inter-trial interval, P = 0.76, Holm-Bonferroni corrected). Red horizontal bar at top indicates timing of optogenetic silencing. Noise bars indicate time of visual stimulus. Grey shaded area indicates post-stimulus period excluded from reactivation analyses. d, Ca2+ activity during stimulus presentation and during the inter-trial interval for control vs. stimulus-inhibition trials (n = 3 mice, two-tailed paired t-test between mean of traces during the specified inter-trial interval, P = 0.19). Red horizontal bar at top indicates timing of optogenetic silencing. Noise bars indicate time of visual stimulus. Grey shaded area indicates post-stimulus period excluded from reactivation analyses. e, Reactivation rate during the inter-trial interval for all stimulus-inhibition trials that were proceeded by a control trial (n = 3 mice, two-tailed linear least-squares regression, Holm-Bonferroni corrected, control trials: P = 0.016, stimulus-inhibition trials: P = 0.38). f, Pupil size-matched reactivation rate during the baseline period before stimulus presentation and during the ITI following stimulus-inhibition trials (n = 3 mice, two-tailed paired t-test, P = 0.018). Times were selected such that the mean pupil size between the two conditions was identical (see Methods). g, Bias index of reactivation content during the inter-trial interval for all stimulus-inhibition trials that were proceeded by a control trial (n = 3 mice, two-tailed linear least-squares regression, Holm-Bonferroni corrected, control trials: P = 0.40, stimulus-inhibition trials: P = 0.66). Here, the bias index is calculated throughout as the bias in reactivation content towards the stimulus presented on the control trial (black vertical line). h, Mean reactivation rate for non-inhibition mice (n = 5 mice) across all trials and for stimulus-inhibition mice (n = 3 mice) across all trials or control (no-inhibition) trials (two-tailed unpaired t-test, Holm-Bonferroni corrected, non-inhibition mice all trials vs. stimulus-inhibition mice all trials: P = 0.11, non-inhibition mice all trials vs. stimulus-inhibition mice control trials: P = 0.019). Data are mean ± SEM. n.s.: not significant; * P < .05; ** P < .01.
Extended Data Fig. 6 Compared to non-inhibition mice, stimulus-inhibition mice exhibit similar stimulus response orthogonalization but higher response magnitudes during control trials.
a, Response similarity shown separately for non-inhibition mice (n = 5 mice) and stimulus-inhibition mice (n = 3 mice; using no-inhibition control trials only). b, Change in response similarity (same as a but after subtracting the mean response similarity in first 3 trials) shown separately for non-inhibition mice (n = 5 mice) and stimulus-inhibition mice (n = 3 mice). c, Heatmap of change in response similarity shown separately for non-inhibition mice (n = 5 mice) and stimulus-inhibition mice (n = 3 mice) for all sessions. d, Response similarity using neurons located in upper layer 2/3 or lower layer 2/3 (n = 8 mice, two-tailed unpaired t-test between the mean of the first or last 3 datapoints of the two traces, Holm-Bonferroni corrected, first: P = 0.74, last: P = 0.85). e, Mean stimulus-evoked activity per trial (across all S1 and S2 trials) averaged across all stimulus-driven neurons shown separately for non-inhibition mice (n = 5 mice) and stimulus-inhibition mice (n = 3 mice; using control trials only). f, Heatmap of mean stimulus-evoked activity shown separately for non-inhibition mice (n = 5 mice) and stimulus-inhibition mice (n = 3 mice) for all sessions. g, Percent of decrease neurons that showed a similar decrease in response to both S1 and S2 (defined as a similar drop in Ca2+ events/second and/or a similar proportional drop in response magnitude to S1 and S2, n = 8 mice). h, Response similarity when using all neurons or when omitting non-differential decrease neurons (n = 8 mice, permutation test between the mean of the first or last 3 datapoints of the two traces, Holm-Bonferroni corrected, first: P = 0.73, last: P = 0.79). Data are mean ± SEM. n.s.: not significant.
Extended Data Fig. 7 Tracking the same neurons across days.
a, Left: example zoom-in of the same subregion of a field of view across six days of imaging. Green, orange, purple, and red boxes highlight the same neurons tracked across all six days. Right: example neurons and masks tracked across six days of imaging, colour-matched to the neurons outlined in the left panel. b, The number of neurons tracked across all six days of imaging (n = 5 non-inhibition mice). c, Change in response similarity from the end of the previous day to the start of the next day across six days of imaging (n = 5 non-inhibition mice, two-tailed paired t-test, P = 0.42). Positive values indicate an increase in response similarity, reflecting a partial relapse in response similarity. Data are mean ± SEM. n.s.: not significant.
Extended Data Fig. 8 Characterization of no-change, increase, and decrease neurons.
a, Mean stimulus-evoked activity across trials shown separately for non-inhibition mice (n = 5 mice) and stimulus-inhibition mice (n = 3 mice, control trials only) for no-change (left), increase (middle), or decrease (right) neuron groups. b, Numbers of neurons that are characterized as no-change, increase, or decrease neurons (n = 8 mice). c, Baseline activity before any stimulus presentation for no-change, increase, and decrease neurons (n = 8 mice, one-way ANOVA, Tukey HSD corrected, P > .05 for all tests). d, Percent of all neurons in visual region LI, POR, P, or LM that were defined as no-change, increase, or decrease neurons (n = 8 mice, one-way ANOVA, Tukey HSD corrected, P > .05 for all tests). e, Percent of all neurons in upper layer 2/3 or in lower layer 2/3 that were characterized as no-change, increase, or decrease neurons (n = 8 mice, two-tailed unpaired t-test, Holm-Bonferroni corrected, P > .05 for all tests). f, Within-group noise correlation (see Methods) of no-change, increase, or decrease neurons (n = 8 mice, one-way ANOVA, Tukey HSD corrected, P > .05 for all tests). g, Response similarity (running correlation between response patterns during neighbouring S1 and S2 trials), plotted in the same manner as the mean trace in Fig. 3g for increase neurons (left) and decrease neurons (right), but shown separately for non-inhibition mice (n = 5 mice) and stimulus-inhibition mice (n = 3 mice). h, Fraction of neurons that increase or decrease their stimulus selectivity (selectivity index: (S1response – S2response) / (S1response + S2response); see Methods) from early to late trials for no-change, increase, or decrease neurons, shown separately for non-inhibition mice (n = 5 mice) and stimulus-inhibition mice (n = 3 mice). i, Cross-correlation between high-pass filtered (see Methods) response similarity and reactivation probability traces shown separately for non-inhibition mice (n = 5 mice) and stimulus-inhibition mice (n = 3 mice). Data are mean ± SEM. n.s.: not significant.
Extended Data Fig. 9 Stimulus reactivations consistently predict future stimulus responses.
a, For each trial, we projected single-trial response patterns (during S1 or S2) and stimulus-specific reactivations during the inter-trial interval (S1R or S2R) onto the axis between early and late stimulus-evoked response patterns within a session (see Fig. 4a, b for additional graphical details). Here, data from a typical example session is shown. b, Same as a but for the mean across all sessions and mice shown separately for non-inhibition mice (n = 5 mice) and stimulus-inhibition mice (n = 3 mice). c, Same as Fig. 4b but shown separately for each of the first six days of imaging per mouse, using all neurons that were tracked across the six days (n = 5 non-inhibition mice). The change in overall y-axis offset per day is not meaningful in this case since each projection uses a different projection axis estimated on each day ‘i’, i = 1–6. d, Same as Fig. 4b but using only neurons from upper layer 2/3 or from lower layer 2/3 (n = 8 mice, permutation test between the mean of the first or last 3 datapoints of the upper layer vs. lower layer traces, Holm-Bonferroni corrected, P > .05 for all tests). e, Same as Fig. 4b but after removing non-differential decrease neurons (see Extended Data Fig. 6g, n = 8 mice, permutation test between the mean of the first or last 3 datapoints of the traces generated using data from all neurons vs. from all neurons after removing non-differential decrease neurons, Holm-Bonferroni corrected, P > .05 for all tests). f, Ratio of mean activity across trials early in each session in increase (top) or decrease (bottom) neurons relative to no-change neurons during S1 presentations vs. S1R reactivation events, and during S2 presentations vs. S2R reactivation events, shown separately for non-inhibition mice (n = 5 mice) and stimulus-inhibition mice (n = 3 mice). g, Stimulus-evoked activity vs. reactivation activity, averaged across all stimulus-driven neurons (n = 8 mice). h, Difference between a neuron’s 1.3x-scaled peri-reactivation activity and its peri-stimulus activity early in each session, averaged across neurons in each group, and shown separately for non-inhibition mice (n = 5 mice) and stimulus-inhibition mice (n = 3 mice). i, Difference between a neuron’s 1.3x-scaled peri-reactivation activity and its peri-stimulus activity early in each session for S1 and S2 trials for neurons that change their stimulus preference (from early to late trials), either from being driven only by S1 to being driven only by S2 (top) or from being driven only by S2 to being driven only by S1 (bottom, n = 8 mice, two-tailed paired t-test, top: P = 3.7 × 10−4, bottom: P = 0.0034). Data are mean ± SEM. n.s.: not significant; ** P < .01; *** P < .001.
Extended Data Fig. 10 Modelling future stimulus responses using only stimulus reactivations.
a, We parametrically varied the plasticity variable γ and measured the error in the modelled stimulus-evoked response patterns vs. actual stimulus-evoked response patterns (mean of the absolute difference between actual and modelled data). The value of 0.2 had the least mean error for both S1 and S2 (n = 8 mice). We used this same value for modelling all sessions and mice. This value is likely an overestimate as we do not consider plasticity that occurs during the stimulus response period. b, Comparison of projection of the actual stimulus-evoked response patterns (dark green/red dots and smoothed trace) with the modelled patterns (light green/pink dots and smoothed trace), projected onto Vs for a single session. c, Comparison of projection of the actual stimulus-evoked response patterns with the modelled patterns, projected onto Vs across all days and mice, shown separately for non-inhibition mice (n = 5 mice) and stimulus-inhibition mice (n = 3 mice). d, Cross-correlation between high-pass filtered actual and modelled projections of stimulus-evoked response patterns shown separately for non-inhibition mice (n = 5 mice) and stimulus-inhibition mice (n = 3 mice, see Methods). e, Response similarity as measured by the correlation between the mean response patterns during nearby S1 and S2 trials, plotted for actual and modelled stimulus responses, shown separately for non-inhibition mice (n = 5 mice) and stimulus-inhibition mice (n = 3 mice). f, Response similarity using modelled data, for increase or decrease neuron groups, shown separately for non-inhibition mice (n = 5 mice) and stimulus-inhibition mice (n = 3 mice). As in Fig. 3g, only decrease neurons show orthogonalization across trials, and this was evident in both sets of mice.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nguyen, N.D., Lutas, A., Amsalem, O. et al. Cortical reactivations predict future sensory responses. Nature 625, 110–118 (2024). https://doi.org/10.1038/s41586-023-06810-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-06810-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.