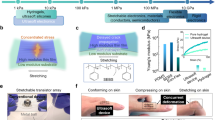
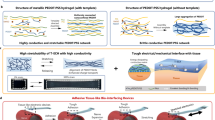
Abstract
Connecting different electronic devices is usually straightforward because they have paired, standardized interfaces, in which the shapes and sizes match each other perfectly. Tissue–electronics interfaces, however, cannot be standardized, because tissues are soft1,2,3 and have arbitrary shapes and sizes4,5,6. Shape-adaptive wrapping and covering around irregularly sized and shaped objects have been achieved using heat-shrink films because they can contract largely and rapidly when heated7. However, these materials are unsuitable for biological applications because they are usually much harder than tissues and contract at temperatures higher than 90 °C (refs. 8,9). Therefore, it is challenging to prepare stimuli-responsive films with large and rapid contractions for which the stimuli and mechanical properties are compatible with vulnerable tissues and electronic integration processes. Here, inspired by spider silk10,11,12, we designed water-responsive supercontractile polymer films composed of poly(ethylene oxide) and poly(ethylene glycol)-α-cyclodextrin inclusion complex, which are initially dry, flexible and stable under ambient conditions, contract by more than 50% of their original length within seconds (about 30% per second) after wetting and become soft (about 100 kPa) and stretchable (around 600%) hydrogel thin films thereafter. This supercontraction is attributed to the aligned microporous hierarchical structures of the films, which also facilitate electronic integration. We used this film to fabricate shape-adaptive electrode arrays that simplify the implantation procedure through supercontraction and conformally wrap around nerves, muscles and hearts of different sizes when wetted for in vivo nerve stimulation and electrophysiological signal recording. This study demonstrates that this water-responsive material can play an important part in shaping the next-generation tissue–electronics interfaces as well as broadening the biomedical application of shape-adaptive materials.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data supporting the findings of this study are available in the paper and its Supplementary Information. Other raw data are available from the corresponding authors upon request. Source data are provided with this paper.
References
Kim, J. et al. Next-generation flexible neural and cardiac electrode arrays. Biomed. Eng. Lett. 4, 95–108 (2014).
Lacour, S. P., Courtine, G. & Guck, J. Materials and technologies for soft implantable neuroprostheses. Nat. Rev. Mater. 1, 16063 (2016).
Bettinger, C. J. Recent advances in materials and flexible electronics for peripheral nerve interfaces. Bioelectron. Med. 4, 6 (2018).
Ampanozi, G. et al. Comparing fist size to heart size is not a viable technique to assess cardiomegaly. Cardiovasc. Pathol. 36, 1–5 (2018).
Hammer, N. et al. Human vagus nerve branching in the cervical region. PLoS One 10, e0118006 (2015).
Parvizi, J. & Kim, G. K. in High Yield Orthopaedics (eds Parvizi, J. & Kim, G. K.) 177–178 (W. B. Saunders, 2010).
Hanlon, J. F., Kelsey, R. J. & Forcinio, H. E. in Handbook of Package Engineering 3rd edn, 59–104 (CRC Press, 1998).
Trznadel, M. Thermally stimulated shrinkage forces in oriented polymers: induction time. Polymer 27, 871–876 (1986).
Kalkan-Sevinc, Z. S. & Strobel, C. T. Material characterization of heat shrinkable film. J. Test. Eval. 43, 1531–1539 (2015).
Work, R. W. A comparative study of the supercontraction of major ampullate silk fibers of orb-web-building spiders (Araneae). J. Arachnol. 9, 299–308 (1981).
Liu, Y., Shao, Z. & Vollrath, F. Relationships between supercontraction and mechanical properties of spider silk. Nat. Mater. 4, 901–905 (2005).
Boutry, C. & Blackledge, T. A. Wet webs work better: humidity, supercontraction and the performance of spider orb webs. J. Exp. Biol. 216, 3606–3610 (2013).
Choi, S. et al. Highly conductive, stretchable and biocompatible Ag–Au core–sheath nanowire composite for wearable and implantable bioelectronics. Nat. Nanotechnol. 13, 1048–1056 (2018).
Zhang, Y. et al. Climbing-inspired twining electrodes using shape memory for peripheral nerve stimulation and recording. Sci. Adv. 5, eaaw1066 (2019).
Xia, Y. et al. A review of shape memory polymers and composites: mechanisms, materials, and applications. Adv. Mater. 33, 2000713 (2021).
Pang, X. et al. Ultralarge contraction directed by light-driven unlocking of prestored strain energy in linear liquid crystal polymer fibers. Adv. Funct. Mater. 30, 2002451 (2020).
Ma, Y. et al. Bioinspired high-power-density strong contractile hydrogel by programmable elastic recoil. Sci. Adv. 6, eabd2520 (2020).
Blacklow, S. O. et al. Bioinspired mechanically active adhesive dressings to accelerate wound closure. Sci. Adv. 5, eaaw3963 (2019).
Lendlein, A. & Langer, R. Biodegradable, elastic shape-memory polymers for potential biomedical applications. Science 296, 1673–1676 (2002).
Mao, Q. et al. Self-contracting oxidized starch/gelatin hydrogel for noninvasive wound closure and wound healing. Mater. Des. 194, 108916 (2020).
Giesa, T. et al. Unraveling the molecular requirements for macroscopic silk supercontraction. ACS Nano 11, 9750–9758 (2017).
Yarger, J. L., Cherry, B. R. & van der Vaart, A. Uncovering the structure–function relationship in spider silk. Nat. Rev. Mater. 3, 18008 (2018).
Wu, Y. et al. Biomimetic supramolecular fibers exhibit water-induced supercontraction. Adv. Mater. 30, 1707169 (2018).
Kim, H. et al. Bio-inspired stretchable and contractible tough fiber by the hybridization of GO/MWNT/polyurethane. ACS Appl. Mater. Interfaces 11, 31162–31168 (2019).
Cera, L. et al. A bioinspired and hierarchically structured shape-memory material. Nat. Mater. 20, 242–249 (2021).
Lang, C. et al. Nanostructured block copolymer muscles. Nat. Nanotechnol. 17, 752–758 (2022).
Yang, C. & Suo, Z. Hydrogel ionotronics. Nat. Rev. Mater. 3, 125–142 (2018).
Liu, Y. et al. Soft and elastic hydrogel-based microelectronics for localized low-voltage neuromodulation. Nat. Biomed. Eng. 3, 58–68 (2019).
Yuk, H., Lu, B. & Zhao, X. Hydrogel bioelectronics. Chem. Soc. Rev. 48, 1642–1667 (2019).
Pan, S. et al. Mechanically interlocked hydrogel–elastomer hybrids for on-skin electronics. Adv. Funct. Mater. 30, 1909540 (2020).
Cai, P. et al. Locally coupled electromechanical interfaces based on cytoadhesion-inspired hybrids to identify muscular excitation-contraction signatures. Nat. Commun. 11, 2183 (2020).
Yuk, H. et al. Skin-inspired hydrogel–elastomer hybrids with robust interfaces and functional microstructures. Nat. Commun. 7, 12028 (2016).
Hubbard, A. M. et al. Hydrogel/elastomer laminates bonded via fabric interphases for stimuli-responsive actuators. Matter 1, 674–689 (2019).
Harada, A. & Kamachi, M. Complex formation between poly(ethylene glycol) and α-cyclodextrin. Macromolecules 23, 2821–2823 (1990).
Liu, S., Rao, Y., Jang, H., Tan, P. & Lu, N. Strategies for body-conformable electronics. Matter 5, 1104–1136 (2022).
Luo, Y. et al. Technology roadmap for flexible sensors. ACS Nano 17, 5211–5295 (2023).
Cogan, S. F. Neural stimulation and recording electrodes. Annu. Rev. Biomed. Eng. 10, 275–309 (2008).
Ganji, M. et al. Scaling effects on the electrochemical stimulation performance of Au, Pt, and PEDOT:PSS electrocorticography arrays. Adv. Funct. Mater. 27, 1703019 (2017).
Boehler, C. et al. Tutorial: guidelines for standardized performance tests for electrodes intended for neural interfaces and bioelectronics. Nat. Protoc. 15, 3557–3578 (2020).
Hukins, D. W. L., Mahomed, A. & Kukureka, S. N. Accelerated aging for testing polymeric biomaterials and medical devices. Med. Eng. Phys. 30, 1270–1274 (2008).
Hoffer, J. A. & Kallesøe, K. in Neural Prostheses for Restoration of Sensory and Motor Function 1st edn (eds Chapin John K. & Moxon, K. A.) 139–170 (CRC Press, 2000).
Song, K.-I. et al. Adaptive self-healing electronic epineurium for chronic bidirectional neural interfaces. Nat. Commun. 11, 4195 (2020).
Navarro, X. et al. A critical review of interfaces with the peripheral nervous system for the control of neuroprostheses and hybrid bionic systems. J. Peripher. Nerv. Syst. 10, 229–258 (2005).
Urbanchek, M. G. et al. Development of a regenerative peripheral nerve interface for control of a neuroprosthetic limb. BioMed Res. Int. 2016, 5726730 (2016).
Vu, P. P. et al. A regenerative peripheral nerve interface allows real-time control of an artificial hand in upper limb amputees. Sci. Transl. Med. 12, eaay2857 (2020).
Liu, J. et al. Intrinsically stretchable electrode array enabled in vivo electrophysiological mapping of atrial fibrillation at cellular resolution. Proc. Natl Acad. Sci. USA 117, 14769–14778 (2020).
Choi, Y. S. et al. Fully implantable and bioresorbable cardiac pacemakers without leads or batteries. Nat. Biotechnol. 39, 1228–1238 (2021).
Li, M. et al. Recent fabrications and applications of cardiac patch in myocardial infarction treatment. VIEW 3, 20200153 (2022).
Montgomery, M. et al. Flexible shape-memory scaffold for minimally invasive delivery of functional tissues. Nat. Mater. 16, 1038–1046 (2017).
Wang, L. et al. Injectable and conductive cardiac patches repair infarcted myocardium in rats and minipigs. Nat. Biomed. Eng. 5, 1157–1173 (2021).
Jiang, Y. et al. A universal interface for plug-and-play assembly of stretchable devices. Nature 614, 456–462 (2023).
Greenspan, L. Humidity fixed points of binary saturated aqueous solutions. J. Res. Natl Bur. Stand. A Phys. Chem. 81A, 89–96 (1977).
Hikima, Y., Morikawa, J. & Hashimoto, T. FT-IR image processing algorithms for in-plane orientation function and azimuth angle of uniaxially drawn polyethylene composite film. Macromolecules 44, 3950–3957 (2011).
Hikima, Y., Morikawa, J. & Kazarian, S. G. Analysis of molecular orientation in polymeric spherulite using polarized micro attenuated total reflection Fourier transform infrared (ATR-FTIR) spectroscopic imaging. Anal. Chim. Acta 1065, 79–89 (2019).
Wunderlich, B. in Macromolecular Physics Vol. 3, chap. 8 (Academic Press, 1980).
Fan, L., Dang, Z., Nan, C.-W. & Li, M. Thermal, electrical and mechanical properties of plasticized polymer electrolytes based on PEO/P(VDF-HFP) blends. Electrochim. Acta 48, 205–209 (2002).
Hess, B., Kutzner, C., van der Spoel, D. & Lindahl, E. GROMACS 4: algorithms for highly efficient, load-Balanced, and scalable molecular simulation. J. Chem. Theory Comput. 4, 435–447 (2008).
Malde, A. K. et al. An automated force field topology builder (ATB) and repository: version 1.0. J. Chem. Theory Comput. 7, 4026–4037 (2011).
Stroet, M. et al. Automated topology builder version 3.0: prediction of solvation free enthalpies in water and hexane. J. Chem. Theory Comput. 14, 5834–5845 (2018).
Asadzadeh, H., Moosavi, A. & Arghavani, J. H. The effect of chitosan and PEG polymers on stabilization of GF-17 structure: A molecular dynamics study. Carbohydr. Polym. 237, 116124 (2020).
Noro, J. et al. Catalytic activation of esterases by PEGylation for polyester synthesis. ChemCatChem 11, 2490–2499 (2019).
Kim, W. et al. A selective membrane-targeting repurposed antibiotic with activity against persistent methicillin-resistant Staphylococcus aureus. Proc. Natl Acad. Sci. USA 116, 16529–16534 (2019).
Humphrey, W., Dalke, A. & Schulten, K. VMD: visual molecular dynamics. J. Mol. Graph. 14, 33–38 (1996).
Berendsen, H. J. C., Grigera, J. R. & Straatsma, T. P. The missing term in effective pair potentials. J. Phys. Chem. 91, 6269–6271 (1987).
Essmann, U. et al. A smooth particle mesh Ewald method. J. Chem. Phys. 103, 8577–8593 (1995).
Sakai, Y. et al. Fabrication and structural analysis of polyrotaxane fibers and films. J. Phys. Condens. Matter 23, 284108 (2011).
Schmalbruch, H. Fiber composition of the rat sciatic nerve. Anat. Rec. 215, 71–81 (1986).
Phinyomark, A., Phukpattaranont, P. & Limsakul, C. Feature reduction and selection for EMG signal classification. Expert Syst. Appl. 39, 7420–7431 (2012).
Thongpanja, S., Phinyomark, A., Phukpattaranont, P. & Limsakul, C. Mean and median frequency of EMG signal to determine muscle force based on time-dependent power spectrum. Elektron. ir Elektrotech. 19, 51–56 (2013).
Acknowledgements
The project was supported by the National Research Foundation (NRF), Prime Minister’s Office, Singapore, under the Singapore Hybrid-Integrated Next-Generation μ-Electronics (SHINE) Centre, Campus of Research Excellence and Technological Enterprise (CREATE), the Smart Grippers for Soft Robotics (SGSR) Program, and the A*STAR MTC Programmatic Funding Scheme (project no. M23L8b0049). G.L. and Z.L. acknowledge support from Shenzhen Science and Technology Program (grant no. KQTD20210811090217009), the Science and Technology Program of Guangdong Province (2022A0505090007) and the National Natural Science Foundation of China (U81927804 and 1913601). G.Z. and H.G. acknowledge support from a start-up grant (002479-00001) from the Nanyang Technological University and the Institute of High Performance Computing, A*STAR, Singapore. Molecular dynamics simulations reported were performed on resources of the National Supercomputing Centre, Singapore. B.H. acknowledges support from the National Natural Science Foundation of China (81971701), the Natural Science Foundation of Jiangsu Province (BK20201352) and the Nanjing Medical University Introduced Talents Scientific Research Start-up Fund (NMUR20190003). We thank R. Du for the discussion on the mechanism and A. L. Chun for reading and editing the paper.
Author information
Authors and Affiliations
Contributions
J.Y. and X.C. designed the project and the experiments. G.Z. and H.G. performed the molecular dynamics simulation and wrote this part of the paper. J.H., Q.T., Q.Y., P.W., Z.C., Y.Li, M.Y., G.L. and Z.L. conducted the in vivo nerve and muscle animal experiments. X.R., Y.Y., W.T. and B.H. did the epicardial recording and minimally invasive implantation. C.W. derived the formula for calculating the confining pressure and wrote this part of the Supplementary Information. L.L. conducted the in vitro cytotoxicity test. J.Y. fabricated all materials and electronics and conducted all other experiments. F.Z., Y.Luo and X.J.L. assisted in material preparation and manuscript preparation. X.Y. and X.W. assisted in structure characterization. W.L. assisted in data processing. Y.J. helped with the electrode designing. J.Y. and X.C. wrote the paper. All authors read and revised the paper.
Corresponding authors
Ethics declarations
Competing interests
J.Y. and X.C. are inventors of the international PCT patent (application no. PCT/SG2022/050764, publication no. WO 2023/075689 A2, filing date 26 October 2022, priority date 26 October 2021, publication date 4 May 2023) filed by the Nanyang Technological University, which covers the WRAP films and WRAP electrode reported in this paper.
Peer review
Peer review information
Nature thanks Nitish Thakor, Zhengzhong Shao and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Comparison of WRAP film with heat shrink film and spider dragline silk.
a, Photographs of heat shrink polymer (polyvinyl chloride) wrapping a tissue-mimicking agarose gel with irregular shape. It contracted to wrap tightly around the hydrogel under 120 °C within seconds. The post-contraction film is much harder (GPa) than its underlying gel (kPa). b, Photographs of WRAP film wrapping an agarose gel with irregular shape. It contracted when dropped water to conformally wrap around the underlying agarose gel and became a soft hydrogel thin film within seconds. c, Schematic of the structure responsible for supercontraction in spider dragline silk. Silk’s supercontraction results from its hierarchical structure consisting of water-penetrable amorphous domains crosslinked by stable β-sheet crystalline domains. The highly oriented polymer chains in the amorphous domains are fixed by hydrogen bonds. Water from wetting breaks these bonds and induces molecular chain recoiling, causing large contractions. d, WRAP film is constructed by stable PEG-α-CD inclusion complex crystalline domains and water-penetrable semi-crystalline PEO domains. WRAP film has oriented PEO crystallites and microporous structure. Break of PEO crystallites by penetrated water causes PEO chain’s recoil and WRAP film’s contraction. Scale bar: 1 cm (a,b); 1 µm (d). Experiments in d were repeated six times with similar results.
Extended Data Fig. 2 Fabrication of WRAP films.
a, Schematic and photographs of stepwise fabrication process of free-standing IC/PEO thin film. b, All-atom MD simulations of spontaneous formation of inclusion complex in solution. As PEG (n = 9) polymer penetrate through the α-CD, their interaction energy decreases. After formation of inclusion complex, the structure remains stable in long time simulation. c, MD simulations of crystalline inclusion complex which is stable and maintains the structure after 100 ns simulation in solution. d, Tensile stress-strain curves for films drawn at 2 mm s−1 show IC/PEO is stiffer (E about 545 MPa) but less stretchable (around 400%) than a pure PEO thin film (370 MPa, 650%). e-f, Relationship between elongation ratio and cycle numbers for different preset stresses (15 MPa to 30 MPa) and crosshead speeds (0.5 mm s−1 to 4 mm s−1) show elongation ratio increased linearly with cycle numbers for all conditions(e). Time-dependent change in elongation ratio under crosshead speeds of 0.5 mm s−1, 2 mm s−1 and 4 mm s−1 shows drawing at 2 mm s−1 is most efficient (f). The higher preset stress and lower drawing speed result in larger elongation ratios’ increase for each cycle. However, the film broke during cold-drawing at a preset stress of 30 MPa and lower drawing speed also means more time is required to reach a certain elongation. Therefore, optimal preset stress and drawing speed for are 25 MPa and 2 mm s−1 respectively. g, Photographs of IC/PEO (elongation 0%) and WRAP films with elongation ratios ranging from 218% to 700% (WRAP-218% to WRAP-700%). Scale bar: 1 cm.
Extended Data Fig. 3 Post-contraction properties of WRAP films.
a, Water content of wet IC/PEO film (elongation ratio, 0%) and PC-WRAP films increased with the elongation ratio increased. b, EIS spectra of wet IC/PEO film and PC-WRAP films (elongation ratio from 218% to 700%) show impedance decreased with elongation ratio increased. However, for elongation ratios > 400%, the decrease in impedance is smaller. All samples had the same exposed area (1.40 cm2) in PBS. c, Stress-strain curves show PC-WRAP films with elongation ratio between 218% and 400% broke at around 600% strain while those with larger elongation ratios broke at lower strains, indicating that stretchability decreased at elongation ratio >400%. d, Surface (right) and cross-section (left) FESEM image of freeze-dried post-contraction wet WRAP hydrogel thin film show typical porous structure. e, Viability/cytotoxicity assay showing the number of live normal human dermal fibroblasts cells after culturing 24 h in conditioned medium (prepared by soaking WRAP-400% in pristine culture medium) is comparable with cells cultured in pristine medium (control). f, Water induces instant shape-adaptive wrapping of WRAP-400% film around a stainless-steel rod. The WRAP film loosely slung around the rod contracts within seconds upon wetting. g, Supercontraction force generated by constrained WRAP-400% shows a transient behavior with 60% decaying within 10 seconds and only 5% remaining after 20 min. Inset: schematic of the test set-up. Testing gauge length was fixed at 0.6 L0, where L0 is the original length of WRAP-400%. Scale bar: 10 µm (d); 100 µm (e); 2 mm (f). Data in a, e are presented as mean ± s.d. from 4 samples. All experiments in d, e were repeated 3 or 4 times with similar results.
Extended Data Fig. 4 Aligned microporous hierarchical structures.
a-b, Surface (a) and Longitudinal section (b) SEM images showing morphology changes of WRAP films. Increasing elongation reveals more pronounced aligned and porous microstructure. c, Surface (left) and longitudinal section (right) SEM images show porous and aligned microstructures disappeared after WRAP-400% contracted under relative humidity of 97%. d-e, Polarized FT-IR spectra for IC/PEO (d) and WRAP-400% film (e). The red and black lines represent electric vectors parallel and perpendicular to elongation direction. For isotropic films, band intensities are similar in both polarizations. WRAP films show PEO bands (e.g., 1342 cm−1, 1281 cm−1) change, α-CD bands (e.g., 1638 cm−1) remain unchanged. f-g, DSC curves (f) and crystallinity of PEO domains (g) in WRAP films with different elongation ratio. As elongation increased, both the half-width (melting range) and area (enthalpy) of the PEO melting peak increased, indicating increased total crystallinity but less uniform crystalline domain size. h, 2D WAXS patterns of IC/PEO and corresponding 1D integrated curves from different scanning azimuthal directions. Isotropic patterns (left) and constant intensities (right) for peak A(100), B(110), D(210), and E(300) belonging to inclusion complex crystallite and peak C(120) and F(032) attributed to PEO crystallites indicate that the IC/PEO structure is isotropic with crystallites oriented randomly. i, Corresponding 1D integrated curves of WRAP-400% films show constant peak intensities (A, B, D, E) of inclusion complex indicating isotropy. Changing intensities (C, F) reveal oriented PEO crystallites. j, Patterns of drawn pure PEO film became anisotropic, constant with anisotropic patterns of WRAP film. k, 2D WAXS patterns of redry PC-WRAP film returned to isotropic. Scale bars: 1 µm (a); 10 µm (b, left), 1 µm (b, right); 1 µm (c, left), 10 µm (c, right). Data in g are presented as mean ± s.d. from three samples. All experiments in a-c were repeated 3-6 times with similar results.
Extended Data Fig. 5 Stability and morphology comparison between undrawn and drawn PEO, α-CD/PEO and IC/PEO films.
a, PC-drawing-400% PEO film dissolved immediately within 10 seconds when wetted. b, PC-drawing-400% α-CD/PEO film (elongation, 400%) gradually dissolved within 1 day in PBS at room temperature. c, PC-WRAP-400% is stable in PBS at room temperature over 2 weeks without collapse. d, Drawing-400% PEO and drawing-400% α-CD/PEO film are semitransparent while WRAP film is white (inset photographs). The SEM results show that WRAP-400% is porous, which was not observed in drawing-400% PEO and drawing-400% α-CD/PEO film. e, Polarized optical microscopy images show the large spherulites in PEO are absent in α-CD/PEO and IC/PEO thin films, and α-CD/PEO is more homogeneous than IC/PEO. f, SEM images of PEO, α-CD/PEO and IC/PEO. The platelet stacking structure in IC/PEO thin film was not observed in PEO and α-CD/PEO thin films. g, Diluting and washing IC/PEO mixture (the mixture before solvent casting) produced a suspension with insoluble precipitates. h, SEM images show that the precipitates have a platelet structure. Left: SEM image of inclusion complex platelets casted on silicon wafer substrate. Right: SEM image of freeze-dried inclusion complex platelets. i, 1-D WAXS curve of inclusion complex platelets indicates that it has typical channel type PEG-α-CD inclusion complex crystalline structure. Scale bar: 1 cm (a,b,c,g); 1 µm (d,f,h); 20 µm (e). All experiments in d-f, h were repeated 3-6 times with similar results.
Extended Data Fig. 6 MD simulations of the interactions between IC/PEO, PEO/PEO, α-CD/PEO in aqueous solution.
a-b, MD simulation of aggregation of PEO-PEO (a), α-CD-PEO (b) respectively in aqueous solution. Water molecules are not shown for clarity. c, Radius of gyration of PEO around inclusion complex (red) increased while that of pure PEO (grey) remained constant. d-f, Hydrogen bonds formation during aggregation of IC-PEO, PEO-PEO, α-CD-PEO respectively in MD simulations. The Distribution of counts of H-bonds obtained from 60−100 ns in simulations show PEO formed more hydrogen bonds with inclusion complex than with PEO or α-CD (f). g-h, Interaction energy change during aggregation of PEO-PEO, α-CD-PEO and IC-PEO respectively in MD simulations. Illustration of the interaction energy change in the aggregation of two molecules, which includes both Van der Waals and coulomb interactions (g). Time evolution of the interaction energy change (h), solid line represents the average from 60-100 ns, with values corresponding to those shown in Fig. 2g.
Extended Data Fig. 7 Structures and in vitro electrochemical properties of WRAP electrodes.
a, Optical microscope images show Au lost its continuity on IC/PEO thin film which isotropically swelled. b-d, Optical microscope (b) and SEM images (c, d) show Au on WRAP-400% films became a continuous mesh of wrinkled micron-sized ribbons because WRAP film contracted along the drawing direction and expanded in the transverse direction. e, Resistance of PC-WRAP-Au slightly increased from about 11 Ω to 13 Ω when undergo strain of 40%, indicating that PC-WRAP-Au film is stretchable. f, Longitudinal sectional SEM image shows spin-coated SEBS penetrated the porous WRAP film to form a seamless mechanical interlocking. g, Optical microscope image show after contraction, wrinkles formed on the SEBS layer of PC-WRAP-SEBS. h, EIS spectra show impedance of WRAP electrode is lower than that of Au-elastomer electrode at low frequency (<4 kHz). Solid plots: impedance; hollow plots: phase angle. i-j, Voltage transients of WRAP electrode (i) and Au-elastomer electrode (j). The pulse currents are 600 μA and 350 μA respectively when the Emc of WRAP electrode and Au-elastomer electrode of the same size are close to −0.6 V. k, Impedance of WRAP-electrode array at 1 kHz remain at about 600 Ω over 5000 cycles of cyclic stretching to 40% strain. l, Impedance of WRAP-electrode array soaked in PBS solution for 2 weeks at 37 °C did not show any obvious changes. m, Accelerated aging test where WRAP electrode is immersed in PBS at 60 °C shows the impedance at 1 kHz more than doubled after 4 days (equivalent to soaking at 37 °C for 20 days). Electrode area exposed to PBS in h-m is around 2.6 mm2. Scale bar: 20 µm (a,b,d); 100 µm (c,g); 5 µm(f); 500 nm (d, inset). All experiments in a-d, f-g were repeated thrice with similar results.
Extended Data Fig. 8 Shape-adaptive implantation, nerve stimulation and compound action potential recording.
a, Waterproof backing and cover for WRAP electrode to prevent premature contraction before installation. b, Schematic of WRAP electrode implementation on nerve. First, place waterproof packaged WRAP electrode beneath nerve. Then remove cover, fold and press the electrode to adhere to form a hollow cylinder around the target nerve. Finally, upon exposure to a drop of water, the WRAP electrode contracted and converted into a soft hydrogel-based electrode, conformally wrapping the nerve. c, Size difference of sciatic nerve and common peroneal nerve in a rat. d, Water-induced contraction of WRAP electrode on the sciatic nerve to form conformal wrap within 2 min. e, Toe movement (extend) of rat induced by common peroneal nerve stimulation using WRAP electrode. f, Photographs of (1) sciatic nerve (around 1.3 mm), (2) common peroneal nerve (PN around 0.5 mm), (3) tibial nerve (TN around 0.7 mm), (4) tibialis anterior muscle (TAM around 13.0 mm) and (5) soleus muscle (SM around 6.0 mm). g, WRAP electrodes (white arrow) conformally wrapped around the TN (3) and SM (5). h, Series of evoked CNAP and CMAP recorded by WRAP electrodes on the PN, TN, TAM, and SM following electrical stimulation of the sciatic nerve using WRAP electrode. Stimulus artifact (red arrow), evoked CNAP (black arrow), evoked CMAP (blue arrow) are shown. i-j, Schematics and photographs show WRAP electrode wrapped conformally around the EDL muscle after being wetted by water (i) while Au-elastomer electrode formed gaps (white arrow) between the electrode and muscle (j). k, Artificial stimulating signals and evoked CMAP signals recorded by WRAP-electrode and Au-elastomer electrode. l, Compared with signals recorded by Au-elastomer electrode, those recorded by WRAP electrode have lower baseline noise. Scale bars: 5 mm (a); 2 mm (c); 1 mm (d,f,i,j); 1 cm (e); g, left 1 mm (g, left), 5 mm (g, right).
Extended Data Fig. 9 Chronic performances of biocompatibility, ENG and RPNI signals recording.
a, Schematic illustrating the experimental setup for ENG (sensory neural signals) recording. WRAP electrode or Au-elastomer electrode were implanted through shape-adaptive contraction or self-locking on sciatic nerves. Electrode leads were subcutaneously tunneled and connected to a headstage fixed to the skull. The headstage was connected to the data acquisition equipment during recording. b, Temperature stimulus was applied by dropping cold (8 °C) and hot (55 °C) water on the hind paw. c, ENG recorded by WRAP electrode implanted on sciatic nerve in response to hot and cold stimuli alternating every 20 seconds. d, H&E-stained images of sciatic nerve cross-section after two weeks of electrode implantation. e, Number of monocytes and lymphocytes per unit area counted from H&E-stained images of the largest nerve fascicles. *P < 0.05; **P < 0.01; NS (P > 0.05), not statistically significant by one-way ANOVA followed by Tukey’s post-hoc test of mean differences. Au-elastomer electrode versus sham control: P(monocytes) = 0.0083, P(lymphocytes) = 0.0031; WRAP-electrode versus sham control: P(monocytes) = 0.43, P(lymphocytes) = 0.11; WRAP electrode versus Au-elastomer electrode: P(monocytes) = 0.037, P(lymphocytes) = 0.040. f, CD68 immunostaining show sciatic nerves fascicles implanted with Au-elastomer overexpress CD68 protein while those with WRAP electrode were similar to sham control. g, Photographs of free soleus muscles from different rats show different size and shape. h, Power spectrum of EMG signals recorded at week 1, 2 and 3. i, Frequency domain features of MNF and MDF calculated from power spectrum of EMG show both MNF and MDF increased at week 2. Scale bar: 200 µm (d); 50 µm (f); 2 mm (g). Data in e and i are presented as mean ± s.d. from three independent rats. All experiments in d, f were repeated three times with similar results.
Extended Data Fig. 10 Epicardial EGM recording and minimally invasive implantation of WRAP electrode.
a, Wrapping a hanging ex vivo chicken heart. Elastomer (SEBS) film larger (top left) or smaller (top right) than the heart failed to wrap the heart. Our shape-adaptive WRAP film (bottom), whose size is initially larger than the heart, contracted and formed a seamless wrap around the heart when wetted. b, Epicardial EGM (top) recorded through a bipolar circular WRAP electrode implanted around the surface of a rat heart and its corresponding spectrogram (bottom). Arrythmia and tachycardia signals were clearly recorded after Aconitine injection at 63 seconds (red dash line). c, Schematic comparing open-chest (left) and minimally invasive implantation (right) of epicardial electrode. Open-chest implantation requires large incision and cutting through the sternum while minimally invasive implantation involves delivering the folded WRAP electrode to the heart through a small incision on the chest guided by a camera. d, Folded WRAP electrode with water-proof protection layer was delivered through a small incision and unfolded in the chest cavity without premature contraction. Scale bar: 1 cm (a).
Supplementary information
Supplementary Information
This file contains Supplementary Notes 1-6, Supplementary Figs. 1-10 and Supplementary References.
41586_2023_6732_MOESM7_ESM.mp4
Supplementary Video 5 WRAP electrodes contract and wrap conformably around the common peroneal nerve and sciatic nerve of rat when wetted during the surgery
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yi, J., Zou, G., Huang, J. et al. Water-responsive supercontractile polymer films for bioelectronic interfaces. Nature 624, 295–302 (2023). https://doi.org/10.1038/s41586-023-06732-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-06732-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.