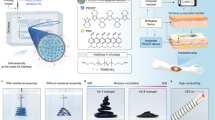
Abstract
Owing to the unique combination of electrical conductivity and tissue-like mechanical properties, conducting polymer hydrogels have emerged as a promising candidate for bioelectronic interfacing with biological systems. However, despite the recent advances, the development of hydrogels with both excellent electrical and mechanical properties in physiological environments is still challenging. Here we report a bi-continuous conducting polymer hydrogel that simultaneously achieves high electrical conductivity (over 11 S cm−1), stretchability (over 400%) and fracture toughness (over 3,300 J m−2) in physiological environments and is readily applicable to advanced fabrication methods including 3D printing. Enabled by these properties, we further demonstrate multi-material 3D printing of monolithic all-hydrogel bioelectronic interfaces for long-term electrophysiological recording and stimulation of various organs in rat models.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data supporting the findings of this study are available within the Article and its Supplementary Information. Additional raw data generated in this study are available from the corresponding authors on reasonable request.
References
Yuk, H., Lu, B. & Zhao, X. Hydrogel bioelectronics. Chem. Soc. Rev. 48, 1642–1667 (2019).
Keplinger, C. et al. Stretchable, transparent, ionic conductors. Science 341, 984–987 (2013).
Yang, C. & Suo, Z. Hydrogel ionotronics. Nat. Rev. Mater. 3, 125–142 (2018).
Dvir, T. et al. Nanowired three-dimensional cardiac patches. Nat. Nanotechnol. 6, 720–725 (2011).
Ohm, Y. et al. An electrically conductive silver–polyacrylamide–alginate hydrogel composite for soft electronics. Nat. Electron. 4, 185–192 (2021).
Tringides, C. M. et al. Viscoelastic surface electrode arrays to interface with viscoelastic tissues. Nat. Nanotechnol. 16, 1019–1029 (2021).
Rivnay, J., Owens, Ri. M. & Malliaras, G. G. The rise of organic bioelectronics. Chem. Mater. 26, 679–685 (2014).
Rivnay, J., Wang, H., Fenno, L., Deisseroth, K. & Malliaras, G. G. Next-generation probes, particles, and proteins for neural interfacing. Sci. Adv. 3, e1601649 (2017).
Gong, J. P., Katsuyama, Y., Kurokawa, T. & Osada, Y. Double‐network hydrogels with extremely high mechanical strength. Adv. Mater. 15, 1155–1158 (2003).
Sun, J.-Y. et al. Highly stretchable and tough hydrogels. Nature 489, 133–136 (2012).
Hua, M. et al. Strong tough hydrogels via the synergy of freeze-casting and salting out. Nature 590, 594–599 (2021).
Liu, C. et al. Tough hydrogels with rapid self-reinforcement. Science 372, 1078–1081 (2021).
Zhao, X. et al. Soft materials by design: unconventional polymer networks give extreme properties. Chem. Rev. 121, 4309–4372 (2021).
Dai, T. et al. Mechanically strong conducting hydrogels with special double-network structure. Synth. Met. 160, 791–796 (2010).
Naficy, S., Oveissi, F., Patrick, B., Schindeler, A. & Dehghani, F. Printed, flexible pH sensor hydrogels for wet environments. Adv. Mater. Technol. 3, 1800137 (2018).
Zhao, Y. et al. Hierarchically structured stretchable conductive hydrogels for high-performance wearable strain sensors and supercapacitors. Matter 3, 1196–1210 (2020).
Wei, H. et al. Orthogonal photochemistry-assisted printing of 3D tough and stretchable conductive hydrogels. Nat. Commun. 12, 2082 (2021).
Javadi, M. et al. Conductive tough hydrogel for bioapplications. Macromol. Biosci. 18, 1700270 (2018).
Yao, B. et al. Ultrahigh‐conductivity polymer hydrogels with arbitrary structures. Adv. Mater. 29, 1700974 (2017).
Liu, Y. et al. Soft and elastic hydrogel-based microelectronics for localized low-voltage neuromodulation. Nat. Biomed. Eng. 3, 58–68 (2019).
Lu, B. et al. Pure PEDOT: PSS hydrogels. Nat. Commun. 10, 1043 (2019).
Liu, Y. et al. Morphing electronics enable neuromodulation in growing tissue. Nat. Biotechnol. 38, 1031–1036 (2020).
Zhao, X. Multi-scale multi-mechanism design of tough hydrogels: building dissipation into stretchy networks. Soft Matter 10, 672–687 (2014).
Feig, V. R., Tran, H., Lee, M. & Bao, Z. Mechanically tunable conductive interpenetrating network hydrogels that mimic the elastic moduli of biological tissue. Nat. Commun. 9, 2740 (2018).
Markvicka, E. J., Bartlett, M. D., Huang, X. & Majidi, C. An autonomously electrically self-healing liquid metal–elastomer composite for robust soft-matter robotics and electronics. Nat. Mater. 17, 618–624 (2018).
Lee, Y. Y. et al. A strain-insensitive stretchable electronic conductor: PEDOT: PSS/acrylamide organogels. Adv. Mater. 28, 1636–1643 (2016).
Lee, S. et al. Nanomesh pressure sensor for monitoring finger manipulation without sensory interference. Science 370, 966–970 (2020).
Qin, D., Xia, Y. & Whitesides, G. M. Soft lithography for micro- and nanoscale patterning. Nat. Protoc. 5, 491–502 (2010).
Yuk, H. et al. 3D printing of conducting polymers. Nat. Commun. 11, 1604 (2020).
Edelman, I. & Leibman, J. Anatomy of body water and electrolytes. Am. J. Med. 27, 256–277 (1959).
Guimarães, C. F., Gasperini, L., Marques, A. P. & Reis, R. L. The stiffness of living tissues and its implications for tissue engineering. Nat. Rev. Mater. 5, 351–370 (2020).
Yuk, H. et al. Dry double-sided tape for adhesion of wet tissues and devices. Nature 575, 169–174 (2019).
Deng, J. et al. Electrical bioadhesive interface for bioelectronics. Nat. Mater. 20, 229–236 (2021).
Yang, Q. et al. Photocurable bioresorbable adhesives as functional interfaces between flexible bioelectronic devices and soft biological tissues. Nat. Mater. 20, 1559–1570 (2021).
Chen, X., Yuk, H., Wu, J., Nabzdyk, C. S. & Zhao, X. Instant tough bioadhesive with triggerable benign detachment. Proc. Natl Acad. Sci. 117, 15497–15503 (2020).
Afanasenkau, D. et al. Rapid prototyping of soft bioelectronic implants for use as neuromuscular interfaces. Nat. Biomed. Eng. 4, 1010–1022 (2020).
Park, S. et al. Adaptive and multifunctional hydrogel hybrid probes for long-term sensing and modulation of neural activity. Nat. Commun. 12, 3435 (2021).
Guo, B. & Ma, P. X. Conducting polymers for tissue engineering. Biomacromolecules 19, 1764–1782 (2018).
Nezakati, T., Seifalian, A., Tan, A. & Seifalian, A. M. Conductive polymers: opportunities and challenges in biomedical applications. Chem. Rev. 118, 6766–6843 (2018).
Seo, B. R. & Mooney, D. J. Recent and future strategies of mechanotherapy for tissue regenerative rehabilitation. ACS Biomater. Sci. Eng. 8, 4639–4642 (2022).
Acknowledgements
The authors thank the Koch Institute Swanson Biotechnology Centre, K. Cormier, and the Histology Core for the technical support and histological processing, and R. Bronson at Harvard Medical School for the histological evaluations. This work is supported by the National Institute of Health (1-R01-HL153857-01, X.Z.).
Author information
Authors and Affiliations
Contributions
H.Y., B.L. and X.Z. developed the concept and materials for the BC-CPH. H.Y. and T.Z. developed the materials and method for the printing-based fabrication and application of the all-hydrogel bioelectronic interfaces. B.L., H.Y., F.H., F.T. and J.X. conducted the electrical and mechanical characterizations of the BC-CPH. T.Z. and H.Y. conducted the electrical and mechanical characterizations of the all-hydrogel bioelectronic interfaces. T.Z., H.Y. and J.W. designed and conducted the in vivo animal studies. H.Y. and H.R. developed the materials and method for the printable bioadhesive. Z.S. and G.G. conducted the AFM phase imaging. H.Y. and X.Z. developed the multi-material printing platform. H.Y. prepared figures with inputs from all authors. H.Y., T.Z. and X.Z. wrote the manuscript with inputs from all authors.
Corresponding authors
Ethics declarations
Competing interests
H.Y. and X.Z. have a financial interest in SanaHeal, a biotechnology company focused on the development of medical devices for surgical sealing and repair. The other authors declare no competing interests.
Peer review
Peer review information
Nature Materials thanks Tal Dvir and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Phase-separation of electrical and mechanical phases in the BC-CPH ink.
a-e, Macroscopic (left) and microscopic (right) images of hydrophilic polyurethane (PU) dissolved in ethanol-water mixed solvent with 90 v/v% (a), 70 v/v% (b), 50 v/v% (c), 30 v/v% (d), and 10 v/v% (e) ethanol concentrations. Green fluorescence corresponds to PU. f–j, Macroscopic (left) and microscopic (right) images of PEDOT:PSS dissolved in ethanol-water mixed solvent with 90 v/v% (f), 70 v/v% (g), 50 v/v% (h), 30 v/v% (i), and 10 v/v% (j) ethanol concentration. k, Macroscopic (left), confocal (middle), and bright-field (right) microscopic images of the BC-CPH ink in ethanol-water mixed solvent with 70 v/v% ethanol concentration. Green fluorescence corresponds to PU. FITC, fluorescein isothiocyanate. Each experiment was repeated independently 3 times.
Extended Data Fig. 2 Long-term stability of the BC-CPH in physiological environment.
a,b, Images (a) and weight (b) of the BC-CPH stored in PBS at 37 °C for 1, 7, 14, 28, 56, 84, and 180 days. c-e, Electrical conductivity (c), ultimate strain (d), and fracture toughness (e) of the BC-CPH stored in PBS at 37 °C. Values in b-e represent the mean and the standard deviation (n = 4; independent samples).
Extended Data Fig. 3 Wet adhesion chemistry of the bioadhesive and rapid sutureless integration to wet tissues.
a, Schematic illustrations for physical crosslinking between the bioadhesive and the target tissue surface by hydrogen bonds. b, Schematic illustrations for covalent crosslinking between the bioadhesive and the target tissue surface by amide bonds. c, Snapshots of sutureless bioadhesive integration of the all-hydrogel bioelectronic interface to a rat sciatic nerve. d, Interfacial toughness of the bioadhesive hydrogel adhered to various rat tissues. Note that tissues underwent cohesive failure for sciatic nerve and spinal cord. Values in d represent the mean and the standard deviation (n = 3; independent experiments).
Extended Data Fig. 4 Electrochemical stability of the all-hydrogel bioelectronic interface.
a–c, Impedance (blue symbols, left axis) and phase angle (red symbols, right axis) vs. frequency plots for one electrode channel in the all-hydrogel bioelectronic interface under varying tensile strain (a), tensile cycle (b), and storage time in PBS at 37 °C (c).
Extended Data Fig. 5 Rat spinal cord stimulation by the all-hydrogel bioelectronic interface.
a, Schematic illustration for rat spinal cord electrophysiological stimulation by the all-hydrogel bioelectronic interface. b, Images of the printed all-hydrogel bioelectronic interface for spinal cord in the overall view (left) and the magnified view of electrodes (right). Different materials are marked with colour overlays in the magnified view. c, Images of the implanted all-hydrogel bioelectronic interface on rat spinal cord. d, e, Images of rat forelimb before (left) and after (middle) electrophysiological stimulation of the spinal cord by the all-hydrogel bioelectronic interface with corresponding EMG recordings (right) on day 0 (d) and day 28 (e) post-implantation. The red-shaded regions in the EMG recordings indicate the stimulation pulses. f, g, Rat forelimb movement distance upon spinal cord stimulations by the all-hydrogel bioelectronic interface at varying stimulation currents on day 0 (f) and day 28 (g) post-implantation. h, Comparison of the rat forelimb movement distance on day 0, day 7, and day 28 post-implantation with stimulation current of 1.5 mA. In box plots (f-h), centre lines represent mean, box limits delineate standard error (SE), and whiskers reflect 5th and 95th percentile (n = 8; independent biological replicates). Statistical significance and p values are determined by two-sided unpaired t-test; *** p ≤ 0.001.
Supplementary information
Supplementary Information
Supplementary Discussions 1 and 2, Supplementary Table 1, Supplementary References and Supplementary Figures 1–33.
Supplementary Video 1
Multi-material 3D printing process of an all-hydrogel bioelectronic interface with the BC-CPH electrodes.
Supplementary Video 2
Sutureless bioadhesive integration of an all-hydrogel bioelectronic interface to a rat sciatic nerve in vivo.
Supplementary Video 3
On-demand detachment of an adhered all-hydrogel bioelectronic interface from a rat sciatic nerve in vivo.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, T., Yuk, H., Hu, F. et al. 3D printable high-performance conducting polymer hydrogel for all-hydrogel bioelectronic interfaces. Nat. Mater. 22, 895–902 (2023). https://doi.org/10.1038/s41563-023-01569-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-023-01569-2
This article is cited by
-
Phase-separated stretchable conductive nanocomposite to reduce contact resistance of skin electronics
Scientific Reports (2024)
-
Microinterfaces in biopolymer-based bicontinuous hydrogels guide rapid 3D cell migration
Nature Communications (2024)
-
An injectable, self-healable, and reusable PEDOT:PSS/PVA hydrogel patch electrode for epidermal electronics
Nano Research (2024)
-
Recent advances in 3D printable conductive hydrogel inks for neural engineering
Nano Convergence (2023)
-
3D-printed PEDOT:PSS for soft robotics
Nature Reviews Materials (2023)