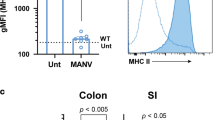
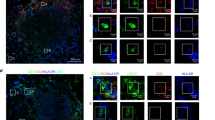
Abstract
All nucleated cells express major histocompatibility complex I and interferon‐γ (IFNγ) receptor1, but an epithelial cell-specific function of IFNγ signalling or antigen presentation by means of major histocompatibility complex I has not been explored. We show here that on sensing IFNγ, colonic epithelial cells productively present pathogen and self-derived antigens to cognate intra-epithelial T cells, which are critically located at the epithelial barrier. Antigen presentation by the epithelial cells confers extracellular ATPase expression in cognate intra-epithelial T cells, which limits the accumulation of extracellular adenosine triphosphate and consequent activation of the NLRP3 inflammasome in tissue macrophages. By contrast, antigen presentation by the tissue macrophages alongside inflammasome-associated interleukin-1α and interleukin-1β production promotes a pathogenic transformation of CD4+ T cells into granulocyte–macrophage colony-stimulating-factor (GM-CSF)-producing T cells in vivo, which promotes colitis and colorectal cancer. Taken together, our study unravels critical checkpoints requiring IFNγ sensing and antigen presentation by epithelial cells that control the development of pathogenic CD4+ T cell responses in vivo.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Source data are provided with this paper.
References
Ivashkiv, L. B. IFNγ: signalling, epigenetics and roles in immunity, metabolism, disease and cancer immunotherapy. Nat. Rev. Immunol. 18, 545–558 (2018).
Dahlhamer, J. M. Prevalence of inflammatory bowel disease among adults aged≥ 18 years—United States, 2015. MMWR Morb. Mortal. Wkly. Rep. 65, 1166–1169 (2016).
Kappelman, M. D., Moore, K. R., Allen, J. K. & Cook, S. F. Recent trends in the prevalence of Crohn’s disease and ulcerative colitis in a commercially insured US population. Dig. Dis. Sci. 58, 519–525 (2013).
Kim, E. R. & Chang, D. K. Colorectal cancer in inflammatory bowel disease: the risk, pathogenesis, prevention and diagnosis. World J. Gastroenterol. 20, 9872–9881 (2014).
Podolsky, D. K. & Isselbacher, K. J. Glycoprotein composition of colonic mucosa. Specific alterations in ulcerative colitis. Gastroenterology 87, 991–998 (1984).
Rhodes, J. M. Unifying hypothesis for inflammatory bowel disease and associated colon cancer: sticking the pieces together with sugar. Lancet 347, 40–44 (1996).
Rabinowitz, K. & Mayer, L. Working out mechanisms of controlled/physiologic inflammation in the GI tract. Immunol. Res. 54, 14–24 (2012).
Platanias, L. C. Mechanisms of type-I-and type-II-interferon-mediated signalling. Nat. Rev. Immunol. 5, 375–386 (2005).
Alspach, E., Lussier, D. M. & Schreiber, R. D. Interferon γ and its important roles in promoting and inhibiting spontaneous and therapeutic cancer immunity. Cold Spring Harb. Perspect. Biol. 11, a028480 (2019).
Castro, F., Cardoso, A. P., Gonçalves, R. M., Serre, K. & Oliveira, M. J. Interferon-gamma at the crossroads of tumor immune surveillance or evasion. Front. Immunol. 9, 847 (2018).
Zhou, Z. et al. Type III interferon (IFN) induces a type I IFN-like response in a restricted subset of cells through signaling pathways involving both the Jak-STAT pathway and the mitogen-activated protein kinases. J. Virol. 81, 7749–7758 (2007).
Jostins, L. et al. Host–microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 491, 119–124 (2012).
Lu, S. et al. Single nucleotide polymorphisms within interferon signaling pathway genes are associated with colorectal cancer susceptibility and survival. PLoS ONE 9, e111061 (2014).
Koroleva, E. P. et al. Citrobacter rodentium-induced colitis: a robust model to study mucosal immune responses in the gut. J. Immunol. Methods 421, 61–72 (2015).
Bouladoux, N., Harrison, O. J. & Belkaid, Y. The mouse model of infection with Citrobacter rodentium. Curr. Protoc. Immunol. 119, 19.15.11–19.15.25 (2017).
Raczynski, A. R. et al. Enteric infection with Citrobacter rodentium induces coagulative liver necrosis and hepatic inflammation prior to peak infection and colonic disease. PLoS ONE 7, e33099 (2012).
Ivanov, I. I. et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139, 485–498 (2009).
Basu, R. et al. Th22 cells are an important source of IL-22 for host protection against enteropathogenic bacteria. Immunity 37, 1061–1075 (2012).
Guy-Grand, D. et al. Origin, trafficking, and intraepithelial fate of gut-tropic T cells. J. Exp. Med. 210, 1839–1854 (2013).
Mortha, A. et al. Microbiota-dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. Science 343, 1249288 (2014).
Pearson, C. et al. ILC3 GM-CSF production and mobilisation orchestrate acute intestinal inflammation. eLife 5, e10066 (2016).
Griseri, T. et al. Granulocyte macrophage colony-stimulating factor-activated eosinophils promote interleukin-23 driven chronic colitis. Immunity 43, 187–199 (2015).
Schnell, A. et al. Stem-like intestinal Th17 cells give rise to pathogenic effector T cells during autoimmunity. Cell 184, 6281–6298.e6223 (2021).
Hamilton, J. A. GM-CSF in inflammation. J. Exp. Med. https://doi.org/10.1084/jem.20190945 (2020).
Hu, Y. et al. Interleukin-1β-induced IRAK1 ubiquitination is required for TH-GM-CSF cell differentiation in T cell-mediated inflammation. J. Autoimmun. 102, 50–64 (2019).
Ronchi, F. et al. Experimental priming of encephalitogenic Th1/Th17 cells requires pertussis toxin-driven IL-1β production by myeloid cells. Nat. Commun. 7, 1–11 (2016).
Sharma, D. & Kanneganti, T.-D. The cell biology of inflammasomes: mechanisms of inflammasome activation and regulation. J. Cell Biol. 213, 617–629 (2016).
Rathinam, V. A., Vanaja, S. K. & Fitzgerald, K. A. Regulation of inflammasome signaling. Nat. Immunol. 13, 333–342 (2012).
Malik, A. & Kanneganti, T. D. Function and regulation of IL-1α in inflammatory diseases and cancer. Immunol. Rev. 281, 124–137 (2018).
Pizzirani, C. et al. Stimulation of P2 receptors causes release of IL-1β–loaded microvesicles from human dendritic cells. Blood 109, 3856–3864 (2007).
Baroni, M. et al. Stimulation of P2 (P2X7) receptors in human dendritic cells induces the release of tissue factor-bearing microparticles. FASEB J. 21, 1926–1933 (2007).
Mariathasan, S. et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440, 228–232 (2006).
Atarashi, K. et al. ATP drives lamina propria TH 17 cell differentiation. Nature 455, 808–812 (2008).
Yona, S. et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 38, 79–91 (2013).
Robson, S. C., Sévigny, J. & Zimmermann, H. The E-NTPDase family of ectonucleotidases: structure function relationships and pathophysiological significance. Purinergic Signal. 2, 409 (2006).
Zadran, S., Amighi, A., Otiniano, E., Wong, K. & Zadran, H. ENTPD5-mediated modulation of ATP results in altered metabolism and decreased survival in gliomablastoma multiforme. Tumor Biol. 33, 2411–2421 (2012).
Feldbrügge, L. et al. Expression of ecto-nucleoside triphosphate diphosphohydrolases-2 and-3 in the enteric nervous system affects inflammation in experimental colitis and Crohn’s disease. J. Crohns Colitis 11, 1113–1123 (2017).
Hashimoto, K., Joshi, S. K. & Koni, P. A. A conditional null allele of the major histocompatibility IA-beta chain gene. Genesis 32, 152–153 (2002).
Huang, S., Meng, Q., Maminska, A. & MacMicking, J. D. Cell-autonomous immunity by IFN-induced GBPs in animals and plants. Curr. Opin. Immunol. 60, 71–80 (2019).
Lim, E. T. et al. Distribution and medical impact of loss-of-function variants in the Finnish founder population. PLoS Genet. 10, e1004494 (2014).
Khanna, T., Hanna, G., Sternberg, M. J. & David, A. Missense3D-DB web catalogue: an atom-based analysis and repository of 4M human protein-coding genetic variants. Hum. Genet. 140, 805–812 (2021).
Ittisoponpisan, S. et al. Can predicted protein 3D structures provide reliable insights into whether missense variants are disease associated? J. Mol. Biol. 431, 2197–2212 (2019).
Huang, B. et al. Mucosal profiling of pediatric-onset colitis and IBD reveals common pathogenics and therapeutic pathways. Cell 179, 1160–1176 (2019).
Friedman, D. J. et al. CD39 deletion exacerbates experimental murine colitis and human polymorphisms increase susceptibility to inflammatory bowel disease. Proc. Natl Acad. Sci. USA 106, 16788–16793 (2009).
Howlader, N. et al. SEER Cancer Statistics Review, 1975–2018 (National Cancer Institute, 2021).
Parang, B., Barrett, C. W. & Williams, C. S. in Gastrointestinal Physiology and Diseases (ed. Ivanov, A. I.) 297–307 (Springer, 2016).
Malik, A. et al. SYK-CARD9 signaling axis promotes gut fungi-mediated inflammasome activation to restrict colitis and colon cancer. Immunity 49, 515–530 (2018).
Ridker, P. M. et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 377, 1119–1131 (2017).
Klück, V. et al. Dapansutrile, an oral selective NLRP3 inflammasome inhibitor, for treatment of gout flares: an open-label, dose-adaptive, proof-of-concept, phase 2a trial. Lancet Rheumatol. 2, e270–e280 (2020).
Lotfi, N. et al. Roles of GM-CSF in the pathogenesis of autoimmune diseases: an update. Front. Immunol. 10, 1265 (2019).
Lee, S. H. et al. Identifying the initiating events of anti-Listeria responses using mice with conditional loss of IFN-γ receptor subunit 1 (IFNGR1). J. Immunol. 191, 4223–4234 (2013).
Madison, B. B. et al. Cis elements of the villin gene control expression in restricted domains of the vertical (crypt) and horizontal (duodenum, cecum) axes of the intestine. J. Biol. Chem. 277, 33275–33283 (2002).
el Marjou, F. et al. Tissue-specific and inducible Cre-mediated recombination in the gut epithelium. Genesis 39, 186–193 (2004).
Malik, A. et al. IL-33 regulates the IgA-microbiota axis to restrain IL-1α–dependent colitis and tumorigenesis. J. Clin. Invest. 126, 4469–4481 (2016).
Marchal-Bressenot, A. et al. Development and validation of the Nancy histological index for UC. Gut 66, 43–49 (2017).
Malik, A., Sharma, D., St Charles, J., Dybas, L. & Mansfield, L. Contrasting immune responses mediate Campylobacter jejuni-induced colitis and autoimmunity. Mucosal Immunol. 7, 802–817 (2014).
Lupfer, C. et al. Receptor interacting protein kinase 2-mediated mitophagy regulates inflammasome activation during virus infection. Nat. Immunol. 14, 480–488 (2013).
Sharma, D., Malik, A., Steury, M. D., Lucas, P. C. & Parameswaran, N. Protective role of β-arrestin2 in colitis through modulation of T-cell activation. Inflam. Bowel Dis. 21, 2766–2777 (2015).
McKenzie, G. J. & Craig, N. L. Fast, easy and efficient: site-specific insertion of transgenes into Enterobacterial chromosomes using Tn 7 without need for selection of the insertion event. BMC Microbiol. 6, 39 (2006).
Acknowledgements
This work was supported by the US National Institutes of Health (R01DK067180) to B.J., the University of Chicago’s Center for Interdisciplinary Study of Inflammatory Intestinal Disorders (C-IID) Pilot & Feasibility Award (NIDDK P30 DK042086) to A.M. and D.S., Crohn’s and Colitis Foundation Career Development Award no. 964209 to A.M. and a G.I. Research Foundation Associates Board Award to A.M. and D.S. Whole-slide scanning was performed by S. Bond and K. Rodriguez in the Integrated Light Microscopy Core at the University of Chicago, which receives financial support from a Cancer Center Support Grant (P30CA014599), RRID:SCR_019197. We thank the University of Chicago Transgenic Mouse Facility (RRID:SCR_019171), especially L. Degenstein, for their assistance with producing the mice used in this study. We thank W. Lisicka, N. Usher, C. Ciszewski, Human Disease and Immune Discovery core (RRID:SCR_022936) and members of the B. Jabri lab at the University of Chicago. The model figure (Extended Data Fig. 11) was created with BioRender.com.
Author information
Authors and Affiliations
Contributions
A.M. and B.J. conceptualized this work. A.M., D.S., R.A.-G., C.W., S.Z. and S.M. developed the methodology. A.M., D.S., S.Z. and S.M. performed the investigation. A.M., D.S., S.M. and B.J. did the formal analysis. A.M. wrote the original draft, and D.S. and B.J. reviewed and edited it. A.M. and B.J. acquired the funding and resources. B.J. supervised the study.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Oliver Pabst and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Progressive increase in IFN-γ signaling and antigen presentation by IECs during colitis.
C57BL/6 J mice were infected with Citrobacter rodentium. (A) Citrobacter colonization in the colon (n = 5 per group) and (B) quantification of Ifng transcript in colon tissue by RT-PCR (n = 4 for d0 and d21, n = 6 for d6 and n = 5 for d12). STAT1 pTyr701 analyses in IECs by (C) immunoblotting, representative of two independent experiments and (D) flow cytometry (n = 3 for d0, n = 6 for d6 and n = 5 for d12 and n = 4 for d21). (E) Quantification of Irf1 transcript in purified IECs (EpCAM+) by RT-PCR (n = 4 for d0, d6 and d21, n = 5 for d12). Flow cytometric analyses for (F and G) MHCI (n = 4 for d0, n = 5 for d6 and d12) and (H and I) MHCII (n = 4 for d0, n = 5 for d6 and d12) in the IECs from colons of mice at indicated day post-infection. (J) Colon histology score in Ifngr1fl/fl and Ifngr1fl/fl VilCre mice. The horizontal bar represents the median, and each symbol and each lane in the immunoblot represents an individual mouse. Data were analyzed by (A, B, D, E, G and I) Brown-Forsthye and Welsch ANOVA tests followed by the Dunnet’s post-hoc test.
Extended Data Fig. 2 Epithelial sensing of IFN-γ orchestrates T cell responses during colitis.
(A-I) Ifngr1fl/fl and Ifngr1fl/fl VilCre mice were infected with Citrobacter rodentium (n = 5 per group). (A-B) Enumeration of I.E. CD4+ T cells and (C-G) evaluation of production of indicated factors by them at indicated days post-infection. Enumeration of (H-L) indicated cells in the epithelium. (M) Comparison of GM-CSF production from ILCs and CD4+ T cells (n = 7 per group) and (N) GM-CSF MFI in ILCs and CD4+ T cells in epithelium of Ifngr1fl/fl VilCre mice at day 12 p.i. (n = 7 per group). (O) The indicated factors were gated globally on the live cell gate and then analyzed for the contribution of CD8αβ+ or CD4+ colonic IE-T cells in their production. Each symbol represents an individual mouse, and the bar indicates the median. Data were analyzed by (A-L) ANOVA followed by the Holm–Sidak post-hoc test or (M-N) Mann-Whitney U test (two-sided). MFI, median fluorescence intensity.
Extended Data Fig. 3 Generation of the ovalbumin-expressing strain of C. rodentium and characterization of ova-specific IE-T cells.
Ovalbumin coding sequence was cloned into the NocI site of pOBX18 plasmid containing the artificial and constitutive Enterobacteriaceae-specific promoter and terminator sequence into the temperature-sensitive transposase 7 attachment site (attTn7) targeting vector (vector described in59) (A) plasmid map and (B) gel picture of the transformed vector. Then, this vector was electroporated into C. rodentium. Growth at permissive temperature along with constitutive expression of the recombination machinery led to the integration of the promoter-ova-terminator sequence into the attTn7 site. Next, the plasmid was cured by subsequent growth at non-permissive temperatures. Site-specific integration was confirmed by (C) PCR and sequencing from the neighboring glmS region of the attTntn7 locus and the expression of ova was confirmed by (D) RT-PCR, representative of two independent clones. (E) Ifngr1fl/fl and Ifngr1fl/fl VilCre mice were infected with ova+ Citrobacter and (F) Ifngrfl/fl VilcreERT2 OvaTg and Ifngrfll+ VilcreERT2 OvaTg mice were infected with wild-type Citrobacter and administered tamoxifen on days 3-6 p.i and evaluated for IFN-γ production from the ova-specific CD4+ IE-T cells (n = 4 per group). (G) Flow cytometric phenotyping of ova-specific CD4+ IE-T cells at day 12 post-infection with ova+ Citrobacter. (H-K) Ifngr1fl/fl, Ifngr1fl/fl VilCreERT2, and Ifngr1fl/fl VilCreERT2 R26LSL Irf1 mice were infected with ova+ Citrobacter rodentium on day 0, injected with tamoxifen at days 3-6 post-infection, and analyzed on day 12 for CD4+ IE-T cells and (I) Citrobacter colonization in the colon (n = 5 per group). (L) Ifngr1fl/fl and Ifngr1fl/flVilCre mice were infected with Citrobacter and treated with isotype or anti-CD4 antibody every 2 days starting at day 7 analyzed for CD4+ T cells on day 12 p.i. (n = 8 per group). The horizontal bar represents the median, and each symbol represents an individual mouse. Data were analyzed by (E and F) Mann-Whitney U test (two-sided) or (H-L) Kruskal-Wallis test followed by Dunn’s post-test.
Extended Data Fig. 4 Epithelial sensing of IFN-γ restrains NLRP3 inflammasome activation in macrophages.
(A-K) Ifngr1fl/fl and Ifngr1fl/fl VilCre mice were infected with Citrobacter rodentium. (A and B) Evaluation of indicated cytokines in colon explants (n = 4 for d0, d6 and n = 8 for d12, per group) and (C) immunoblotting for caspase-1 p10 in colon lysates, representative of two independent experiments. (D) Evaluation of IL-1α in colon explants, (E-F) Colon histology analyses and (G) Citrobacter colonization after isotype (n = 8), αβ-ATP (n = 8), apyrase (n = 6), MCC950 (n = 6) or anti-IL1R (n = 6) treatment. (H-K) Ifngr1fl/fl and Ifngr1fl/fl VilCre mice were infected and given 500ug of anti-CSF1R or isotype control antibody on days 7, 9 and 11 p.i and sacrificed on day 12 p.i. (n = 8 per group). (H) Flow cytometric plot for colon macrophages from Ifngr1fl/fl VilCre mice treated with anti-CSF1R or isotype control antibody. (I) Evaluation of indicated cytokines in colon explants. (J) Flow-cytometric analyses of CD4+ IE-T cells and GM-CSF production from them and (K) colon length at day 12 p.i. Data were analyzed by Brown-Forsythe and Welch’s ANOVA followed by Dunnett’s post-test. Scale bar = 100 um.
Extended Data Fig. 5 Antigen presentation by CX3CR1 + APCs promote TH1 immunity.
H2abfl/fl and H2abΔiCX3CR1 mice were infected with Citrobacter, injected with tamoxifen on days 3–6 and sacrificed on day 12 p.i. (A) Flow cytometric analyses of MHCII expression in CD172a+ cells from the MLNs of H2abfl/fl and H2abΔiCX3CR1 mice (n = 9 per group). (B) enumeration of GM-CSF- IL-17A+ CD4+ IE-T cells from the MLNs of H2abfl/fl»IfngrΔiIEC and H2abΔiCX3CR1»IfngrΔiIEC chimeric mice after treatments described in Fig. 3g at day 12 p.i. (n = 7 per group). (C) H2abfl/fl and H2abΔiCX3RC1 mice were treated with tamoxifen for 4 consecutive days, followed by infection with Citroova 3 days after the last injection. Mice were injected with CTV-labelled 1 × 106 Rag-OTII cells on day 5 p.i., treated with 20ug of FTY720 i.p. on days 6-8 and sacrificed on day 9 (n = 6 per group). (C) Experimental scheme (D) OTII cells from MLNs were analyzed for T-bet induction. Data were analyzed by (A and D) by Mann-Whitney U test (two-sided) or (B) Kruskal-Wallis test followed by Dunn’s post-test.
Extended Data Fig. 6 Epithelial sensing of IFN-γ promotes eATPase production from IE-T cells.
(A-I) Ifngr1fl/fl and Ifngrfl/fl VilCre mice were infected with Citrobacter rodentium. (A and B) RT-PCR analyses for indicated factors in colon epithelial tissue fraction (n = 4 for d0, d6 per group and n = 8 for Ifngr1fl/fl and n = 6 for Ifngr1fl/fl VilCre for d12). (C, E-I) flow cytometric (n = 3 for d0, n = 5 for d6 and d12 per group) and (D) immunofluorescence evaluation of CD39 expression, representative of two independent experiments and (G) RT-PCR analyses of Entpd5 expression from the purified IE-T cells and IECs (n = 6 per group). (I) Ifngr1fl/fl and Ifngr1fl/fl VilCre mice were infected with ova+ Citrobacter and the ova-specific CD8αβ+ and CD4+ IE-T cells were evaluated for CD39 expression and (J) comparison of GM-CSF and CD39 producing CD4+ colonic IE-T cells in Ifngr1fl/fl mice at day 12 p.i. (K-M) Ifngr1fl/fl and Ifngr1fl/fl VilCre mice were infected with Citrobacter rodentium and treated with 250ug isotype or CD8β-depleting antibodies every 2 days after the infection and sacrificed on day 12 p.i. (n = 8 per group). (K) FACS plot for CD8+ IE-T cells from mice given isotype or CD8β-depleting antibodies. (L) Colon explants were analyzed for indicated analytes and (M) colon length measurement. (N) Ifngr1fl/fl, Ifngr1fl/fl VilCreERT2 and Ifngr1fl/fl VilCreERT2 R26LSLIrf1 mice were infected with Citrobacter rodentium on day 0, injected with tamoxifen at days 3–6 post-infection and analyzed on day 12 for CD39 expression from CD8αβ+ and CD4+ IE-T cells (n = 5 per group). The horizontal bar represents the median, and each symbol represents an individual mouse. Data were analyzed by (A, B) by Brown-Forsythe and Welch’s ANOVA followed by Dunnett’s post-test or (E-I) Mann-Whitney U test (two-sided) or (L-N) Kruskal-Wallis test followed by Dunn’s post-test. Scale bar = 100 um.
Extended Data Fig. 7 Epithelial IFN-γ signaling controls antigen presentation by IECs.
Flow cytometric enumeration of MHCII (A and C) or MHCI (B) or SIINFEKL-MHCI complex (D-E) on the surface of the colonic IECs after infection with CitroWT (A and E) or CitroOva (B-D) at indicated day p.i. (A) or on day 12 p.i. (n = 5 per group). (B-E). Tamoxifen treatment was applied to induce the expression of the transgenes (B-E) at days 3-6 p.i. (F) Ifngr1fl/fl and Ifngr1fl/flVilCre mice were infected with Citrobacter and treated with anti-CD4 (n = 6) or isotype antibodies (n = 4) every 2 days starting at day 6 and sacrificed on day 12 p.i. The horizontal bar represents the median, and each symbol represents an individual mouse. Data were analyzed by (A) by ANOVA followed by Holm-Sidak post-test or (B-D and F) Kruskal-Wallis test followed by Dunn’s post-test or (E) Mann-Whitney U test (two-sided).
Extended Data Fig. 8 Antigen presentation via MHCI by IECs promotes eATPase production from CD8ab+ IE-T cells.
(A) Design and genotyping of B2mfl/fl mouse, representative of two independent lines. (B-K) B2mfl/fl and B2mfl/flVilCreERT2 mice were infected with ova-expressing Citrobacter on day 0, treated with tamoxifen on days 3–6 and sacrificed on day 12. Colonic IECs on day 12 were analyzed for (B) MHCI expression (n = 5 for B2mfl/fl and n = 7 B2mfl/flVilCreERT2) and (C) IE-T cells for CD39 expression (n = 5 per group) (D-E) (n = 3 for d0 and n = 5 for d6, d12 per group) by flow cytometry. (F-I) quantification of indicated analytes from colon explants (n = 8 per group) and (J) GM-CSF production from the ova-specific CD4+ IE-T cells and (K) Citrobacter colonization in the colon at day 12 p.i. (n = 5 per group). (L-N) B2mfl/fl and B2mfl/flVilCreERT2 mice were infected with ova-expressing Citrobacter on day 0, treated with tamoxifen on days 3–6, injected with anti-NK1.1 or isotype antibodies on days 2, 4, 6, 8 and 10 and sacrificed on day 12 (n = 5 per group). (L) IFN-γ production from NK1.1+ TCRαβ− TCRγδ− cells (M) CD45+CD49b+ cells and (N) colon length at day 12. The horizontal bar represents the median, and each symbol represents an individual mouse. Data were analyzed by (B, D, E and N) ANOVA test followed by Sidak’s post-test or (C, F-L) Kolmogorov-Smirnov test.
Extended Data Fig. 9 Antigen presentation by IECs promotes eATPase production from CD4+ IE-T cells.
H2abfl/fl and H2abfl/flVilCreERT2 mice were infected with ova-expressing Citrobacter on day 0, treated with tamoxifen on days 3-6, and sacrificed on day 12 (n = 5-8 per group). (A) Colonic IECs and myeloid cells were analyzed for MHCII expression on day 12 and (C-F) IE-T cells for CD39 expression by flow cytometry (n = 5 per group). (G) Entpd5 expression by RT-PCR after purification (n = 6 per group). (H-K) quantification of indicated analytes from colon explants (n = 8 pe group). (L-M) GM-CSF production from the CD4+ IE-T cells (n = 5 per group). (N) colon length and (O) Citrobacter colonization in the colon at day 12 p.i. (n = 8 per group). The horizontal bar represents the median, and each symbol represents an individual mouse. Data were analyzed by (A-O) Mann-Whitney U test (two-sided).
Extended Data Fig. 10 Antigen presentation by IECs restrains pathogenic CD4 T cells that promote chemical colitis.
(A-I) Ifngr1fl/fl and Ifngr1fl/flVilCre were treated with 1% DSS for 9 days followed by regular drinking water and sacrificed on day 12. (A) Expression of MHCI and (B) MHCII on the epithelial cell surface (n = 6 per group). Expression of CD39 on (C) CD8αβ+ and (D) CD4+ IE-T cells (n = 5 per group). (E) Quantification of ATP in the colon explants (n = 8 per group) and (F) production of GM-CSF from the CD4+ IE-T cells at day 12 (n = 5 per group). (G) Enumeration of neutrophils in the epithelium and (H) Bodyweight change and (I) colon length (n = 6 per group) and representative picture at day 12. (J-M) B2mfl/fl and B2mfl/flVilCreERT2 mice (n = 5 per group) were treated with 1% DSS for 9 days followed by regular drinking water and sacrificed on day 12. Mice were also treated with tamoxifen on days 3-6. (N) Colon lengths from Ifngr1fl/fl and Ifngr1fl/flVilCre mice treated isotype (n = 10), α-CD4 (n = 8), α-GM-CSF (n = 8), αβ-ATP (n = 10), apyrase (n = 10), MCC950 (n = 10) or α-IL1R (n = 10) during DSS administration and sacrificed on day 12. Each symbol represents an individual mouse, and the bar represents the median except for (H) where each dot indicated the mean and bar indicates the SEM. Data were analyzed by (A-G, I-M) Mann-Whitney U test (two-sided) or (H) ANOVA followed by Sidak post-test or (N) Kruskal-Wallis test followed by Dunn’s post-test.
Extended Data Fig. 11 Model.
Upon sensing IFN-γ, colonic epithelial cells productively present pathogen-derived and self-antigens to the cognate intraepithelial T cells, critically situated at the epithelial barrier. Antigen presentation by the epithelial cells induces extracellular adenosine triphosphatase (ATPase) expression in the cognate intraepithelial T cells, which limits accumulation of extracellular ATP and resultant activation of the NLRP3 inflammasome in macrophages resident in the tissue. In contrast, macrophage antigen presentation alongside inflammasome-associated interleukin 1 alpha (IL-1α) and IL-1β production elicits pathogenic transformation of CD4-positive T cells into granulocyte-macrophage colony stimulating factor (GM-CSF)-producing T cells in vivo, promoting colitis and colorectal carcinogenesis. The figure was created using BioRender (https://biorender.com).
Supplementary information
Supplementary Information
This file contains the uncropped blots and gels.
Source data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Malik, A., Sharma, D., Aguirre-Gamboa, R. et al. Epithelial IFNγ signalling and compartmentalized antigen presentation orchestrate gut immunity. Nature 623, 1044–1052 (2023). https://doi.org/10.1038/s41586-023-06721-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-06721-1
This article is cited by
-
Distal colonocytes targeted by C. rodentium recruit T-cell help for barrier defence
Nature (2024)
-
Interferon-γ sensing by epithelial cells tames gut inflammation
Nature Immunology (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.