Abstract
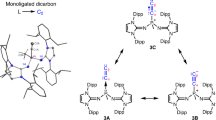
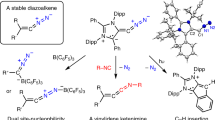
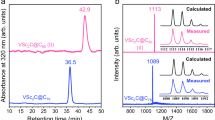
The chemistry of carbon is governed by the octet rule, which refers to its tendency to have eight electrons in its valence shell. However, a few exceptions do exist, for example, the trityl radical (Ph3C∙) (ref. 1) and carbocation (Ph3C+) (ref. 2) with seven and six valence electrons, respectively, and carbenes (R2C:)—two-coordinate octet-defying species with formally six valence electrons3. Carbenes are now powerful tools in chemistry, and have even found applications in material and medicinal sciences4. Can we undress the carbene further by removing its non-bonding electrons? Here we describe the synthesis of a crystalline doubly oxidized carbene (R2C2+), through a two-electron oxidation/oxide-ion abstraction sequence from an electron-rich carbene5. Despite a cumulenic structure and strong delocalization of the positive charges, the dicoordinate carbon centre maintains significant electrophilicity, and possesses two accessible vacant orbitals. A two-electron reduction/deprotonation sequence regenerates the parent carbene, fully consistent with its description as a doubly oxidized carbene. This work demonstrates that the use of bulky strong electron-donor substituents can simultaneously impart electronic stabilization and steric protection to both vacant orbitals on the central carbon atom, paving the way for the isolation of a variety of doubly oxidized carbenes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Crystallographic data for this paper (CCDC 2245611–2245613, 2267007, 2267374) are available free of charge via the Cambridge Crystallographic Data Centre. Non-crystallographic data are provided in the Supplementary information file.
References
Gomberg, M. An instance of trivalent carbon: triphenylmethyl. J. Am. Chem. Soc. 22, 757–771 (1900).
Grützmacher, H. & Marchand, C. M. Heteroatom stabilized carbenium ions. Coord. Chem. Rev. 163, 287–344 (1997).
Bourissou, D., Guerret, O., Gabbaï, F. P. & Bertrand, G. Stable carbenes. Chem. Rev. 100, 39–91 (2000).
Bellotti, P., Koy, M., Hopkinson, M. N. & Glorius, F. Recent advances in the chemistry and applications of N-heterocyclic carbenes. Nat. Rev. Chem. 5, 711–725 (2021).
Loh, Y. K., Melaimi, M., Munz, D. & Bertrand, G. An air-stable “masked” bis(imino)carbene: a carbon-based dual ambiphile. J. Am. Chem. Soc. 145, 2064–2069 (2023).
Igau, A., Grützmacher, H., Baceiredo, A. & Bertrand, G. Analogous α,α′-bis-carbenoid triply bonded species: synthesis of a stable λ3-phosphinocarbene−λ3-phosphaacetylene. J. Am. Chem. Soc. 110, 6463–6466 (1988).
Arduengo, A. J. III, Harlow, R. L. & Kline, M. A stable crystalline carbene. J. Am. Chem. Soc. 113, 361–363 (1991).
Parker, V. D. & Bethell, D. Carbene cation radicals: the kinetics of their formation from diazoalkane cation radicals and their reactions. J. Am. Chem. Soc. 109, 5066–5072 (1987).
Bethell, D. & Parker, V. D. In search of carbene ion radicals in solution: reaction pathways and reactivity of ion radicals of diazo compounds. Acc. Chem. Res. 21, 400–407 (1988).
Bally, T., Matzinger, S. & Truttman, L. Diphenyl carbene cation: electronic and molecular structure. J. Am. Chem. Soc. 115, 7007–7008 (1993).
Stoub, D. & Goodman, G. V. Diarylcarbene cation radicals: generation and chemical reactivity in solution. J. Am. Chem. Soc. 119, 11110–11111 (1997).
Ramnial, T., McKenzie, I., Gorodetsky, B., Tsang, E. M. W. & Clyburne, J. A. C. Reactions of N-heterocyclic carbenes (NHCs) with one-electron oxidants: possible formation of a carbene cation radical. Chem. Commun. 1054–1055 (2004).
Dong, Z. et al. SET processes in Lewis acid−base reactions: the tritylation of N-heterocyclic carbenes. Chem. Sci. 11, 7615–7618 (2020).
Shaikh, A. C., Veleta, J. M., Moutet, J. & Gianetti, T. L. Trioxatriangulenium (TOTA+) as a robust carbon-based Lewis acid in frustrated Lewis pair chemistry. Chem. Sci. 12, 4841–4849 (2021).
Maiti, A. et al. Disclosing cyclic(alkyl)(amino)carbenes as one-electron reductants: synthesis of acyclic(amino)(aryl)carbene-based Kekulé diradicaloids. Chem. Eur. J. 28, e202104567 (2022).
Song, H. & Lee, E. Revisiting the reaction of IPr with tritylium: an alternative mechanistic pathway. Chem. Eur. J. 29, e202203364 (2023).
Zhang, Q., Lei, H., Zhou, C.-Y. & Wang, C. Construction of N-polyheterocycles by N-heterocyclic carbene catalysis via a regioselective intramolecular radical addition/cyclization cascade. Org. Lett. 24, 4615–4619 (2022).
Liu, L., Zhang, Q. & Wang, C. Redox-neutral generation of iminyl radicals by N-heterocyclic carbene catalysis: rapid access to phenanthridines from vinyl azides. Org. Lett. 24, 5913–5917 (2022).
Courtenay, S., Mutus, J. Y., Schurko, R. W. & Stephan, D. W. The extended borinium cation: [(tBu3PN)2B]+. Angew. Chem. Int. Ed. 41, 498–501 (2002).
Shoji, Y., Tanaka, N., Mikami, K., Uchiyama, M. & Fukushima, T. A two-coordinate boron cation featuring C−B+−C bonding. Nat. Chem. 6, 498–503 (2014).
Bamford, K. L., Qu, Z.-W. & Stephan, D. W. Activation of H2 and Et3SiH by the borinium cation [Mes2B]+: avenues to cations [MesB(μ-H)2(μ-Mes)BMes]+ and [H2B(μ-H)(μ- Mes)B(μ-Mes)(μ-H)BH2]+. J. Am. Chem. Soc. 141, 6180–6184 (2019).
Franz, D. & Inoue, S. Cationic complexes of boron and aluminum: an early 21st century viewpoint. Chem. Eur. J. 25, 2898–2926 (2018).
Piers, W. E., Bourke, S. C. & Conroy, K. C. Borinium, borenium, and boronium ions: synthesis, reactivity, and applications. Angew. Chem. Int. Ed. 44, 5016–5036 (2005).
Ochiai, T., Franz, D. & Inoue, S. Applications of N-heterocyclic imines in main group chemistry. Chem. Soc. Rev. 45, 6327–6344 (2016).
Goettel, J. T., Gao, H., Dotzauer, S. & Braunschweig, H. MeCAAC=N−: a cyclic (alkyl)(amino)carbene imino ligand. Chem. Eur. J. 26, 1136–1143 (2020).
Huynh, S. et al. Cyclic alkyl(amino)iminates (CAAIs) as strong 2σ,4π-electron donor ligands for the stabilisation of boranes and diboranes(4): a synthetic and computational study. Dalton Trans. 52, 3869–3876 (2023).
Pal, S., Manae, M. A., Khade, V. V. & Khan, S. Reactivity of N-heterocyclic carbene, 1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene, towards heavier halogens (Br2 and I2). J. Indian Chem. Soc. 95, 765–770 (2018).
Loh, Y. K., Fuentes, M. Á., Vasko, P. & Aldridge, S. Successive protonation of an N-heterocyclic imine derived carbonyl: superelectrophilic dication versus masked acylium ion. Angew. Chem. Int. Ed. 57, 16559–16563 (2018).
Loh, Y. K. et al. An acid-free anionic oxoborane isoelectronic with carbonyl: facile access and transfer of a terminal B=O double bond. J. Am. Chem. Soc. 141, 8073–8077 (2019).
Januszewski, J. A. & Tykwinski, R. R. Synthesis and properties of long [n]cumulenes (n ≥ 5). Chem. Soc. Rev. 43, 3184–3203 (2014).
Pinter, P. & Munz, D. Controlling möbius-type helicity and the excited-state properties of cumulenes with carbenes. J. Phys. Chem. A 124, 10100–10110 (2020).
Couchman, S. A., Wilson, D. J. D. & Dutton, J. L. Is the perfluorinated trityl cation worth a revisit? A theoretical study on the Lewis acidities and stabilities of highly halogenated trityl derivatives. Eur. J. Org. Chem. 3902–3908 (2014).
Jupp, A. R., Johnstone, T. C. & Stephan, D. W. The global electrophilicity index as a metric for Lewis acidity. Dalton Trans. 47, 7029–7035 (2018).
Zhou, J., Liu, L. L., Cao, L. L. & Stephan, D. W. A phosphorus Lewis super acid: η5-pentamethylcyclopentadienyl phosphorus dication. Chem 4, 2699–2708 (2018).
Mehlmann, P., Witteler, T., Wilm, L. F. B. & Dielmann, F. Isolation, characterization and reactivity of three-coordinate phosphorus dications isoelectronic to alanes and silylium cations. Nat. Chem. 11, 1139–1143 (2019).
Olah, G. et al. Bridgehead adamantyl, diamantyl, and related cations and dications. J. Am. Chem. Soc. 107, 2764–2772 (1985).
Kato, T. & Reed, C. A. Putting tert-butyl cation in a bottle. Angew. Chem. Int. Ed. 43, 2908–2911 (2004).
Légaré, M.-A., Pranckevicius, C. & Braunschweig, H. Metallomimetic chemistry of boron. Chem. Rev. 119, 8231–8261 (2019).
Weetman, S. & Inoue, S. The road travelled: after main-group elements as transition metals. ChemCatChem 10, 4213–4228 (2018).
Martin, D., Melaimi, M., Soleilhavoup, M. & Bertrand, G. Stable singlet carbenes as mimics for transition metal centers. Chem. Sci. 2, 389–399 (2011).
Lipshultz, J. M., Li, G. & Radosevich, A. T. Main group redox catalysis of organopnictogens: vertical periodic trends and emerging opportunities in group 15. J. Am. Chem. Soc. 143, 1699–1721 (2021).
Olah, G. A. My search for carbocations and their role in chemistry (Nobel lecture). Angew. Chem. Int. Ed. 34, 1393–1405 (1995).
Acknowledgements
This work was supported by the NSF (grant no. CHE-2246948). We thank A*STAR for a postdoctoral fellowship for Y.K.L. We acknowledge the scientific support and HPC resources provided by the Erlangen National High Performance Computing Center (NHR@FAU) of the Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU). The hardware is funded by the German Research Foundation (DFG). We thank F. F. Mulks for helpful discussions.
Author information
Authors and Affiliations
Contributions
Y.K.L. conceived and performed the synthetic experiments. M.M. and M.G. performed the X-ray crystallographic analyses. D.M. performed the computational work. G.B. supervised the project. The manuscript was written by Y.K.L., M.M. and G.B.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–30, Tables 1–10, Methods and references.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Loh, Y.K., Melaimi, M., Gembicky, M. et al. A crystalline doubly oxidized carbene. Nature 623, 66–70 (2023). https://doi.org/10.1038/s41586-023-06539-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-06539-x
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.