Abstract
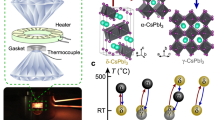
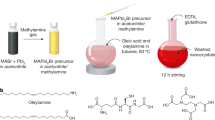
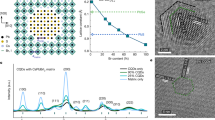
Although high-entropy materials are excellent candidates for a range of functional materials, their formation traditionally requires high-temperature synthetic procedures of over 1,000 °C and complex processing techniques such as hot rolling1,2,3,4,5. One route to address the extreme synthetic requirements for high-entropy materials should involve the design of crystal structures with ionic bonding networks and low cohesive energies. Here we develop room-temperature-solution (20 °C) and low-temperature-solution (80 °C) synthesis procedures for a new class of metal halide perovskite high-entropy semiconductor (HES) single crystals. Due to the soft, ionic lattice nature of metal halide perovskites, these HES single crystals are designed on the cubic Cs2MCl6 (M=Zr4+, Sn4+, Te4+, Hf4+, Re4+, Os4+, Ir4+ or Pt4+) vacancy-ordered double-perovskite structure from the self-assembly of stabilized complexes in multi-element inks, namely free Cs+ cations and five or six different isolated [MCl6]2– anionic octahedral molecules well-mixed in strong hydrochloric acid. The resulting single-phase single crystals span two HES families of five and six elements occupying the M-site as a random alloy in near-equimolar ratios, with the overall Cs2MCl6 crystal structure and stoichiometry maintained. The incorporation of various [MCl6]2– octahedral molecular orbitals disordered across high-entropy five- and six-element Cs2MCl6 single crystals produces complex vibrational and electronic structures with energy transfer interactions between the confined exciton states of the five or six different isolated octahedral molecules.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the article and its Supplementary Information, and are available from the corresponding author on reasonable request. The crystallographic information files have also been deposited in the Inorganic Crystal Structure Database under reference nos. CSD 2216614–2216620, 2216624, 2216626, 2216627 and 2216636–2216643. These data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/, or by emailing data_request@ccdc.cam.ac.uk.
References
Liu, D. et al. Exceptional fracture toughness of CrCoNi-based medium- and high-entropy alloys at 20 kelvin. Science 378, 978–983 (2022).
Jin, K. et al. Tailoring the physical properties of Ni-based single-phase equiatomic alloys by modifying the chemical complexity. Sci. Rep. 6, 20159 (2016).
Zhang, R.-Z., Gucci, F., Zhu, H., Chen, K. & Reece, M. J. Data-driven design of ecofriendly thermoelectric high-entropy sulfides. Inorg. Chem. 57, 13027–13033 (2018).
Stolze, K., Tao, J., von Rohr, F. O., Kong, T. & Cava, R. J. Sc–Zr–Nb–Rh–Pd and Sc–Zr–Nb–Ta–Rh–Pd high-entropy alloy superconductors on a CsCl-type lattice. Chem. Mater. 30, 906–914 (2018).
Nguyen, T. X., Liao, Y., Lin, C., Su, Y. & Ting, J. Advanced high entropy perovskite oxide electrocatalyst for oxygen evolution reaction. Adv. Funct. Mater. 31, 2101632 (2021).
Roychowdhury, S., Ghosh, T., Arora, R., Waghmare, U. V. & Biswas, K. Stabilizing n‐type cubic GeSe by entropy‐driven alloying of AgBiSe2: ultralow thermal conductivity and promising thermoelectric performance. Angew. Chem. Int. Ed. Engl. 130, 15387–15391 (2018).
Deng, Z. et al. Semiconducting high-entropy chalcogenide alloys with ambi-ionic entropy stabilization and ambipolar doping. Chem. Mater. 32, 6070–6077 (2020).
Luo, Y. et al. High thermoelectric performance in the new cubic semiconductor AgSnSbSe3 by high-entropy engineering. J. Am. Chem. Soc. 142, 15187–15198 (2020).
Wu, Z., Bei, H., Pharr, G. M. & George, E. P. Temperature dependence of the mechanical properties of equiatomic solid solution alloys with face-centered cubic crystal structures. Acta Mater. 81, 428–441 (2014).
Rost, C. M., Rak, Z., Brenner, D. W. & Maria, J. Local structure of the MgxNixCoxCuxZnxO (x=0.2) entropy‐stabilized oxide: an EXAFS study. J. Am. Ceram. Soc. 100, 2732–2738 (2017).
Jiang, S. et al. A new class of high-entropy perovskite oxides. Scr. Mater. 142, 116–120 (2018).
Jiang, B. et al. High-entropy-stabilized chalcogenides with high thermoelectric performance. Science 371, 830–834 (2021).
Rombach, F. M., Haque, S. A. & Macdonald, T. J. Lessons learned from spiro-OMeTAD and PTAA in perovskite solar cells. Energy Environ. Sci. 14, 5161–5190 (2021).
Gao, M. et al. The making of a reconfigurable semiconductor with a soft ionic lattice. Matter 4, 3874–3896 (2021).
Lai, M. et al. Intrinsic anion diffusivity in lead halide perovskites is facilitated by a soft lattice. Proc. Natl Acad. Sci. USA 115, 11929–11934 (2018).
Protesescu, L. et al. Nanocrystals of cesium lead halide perovskites (CsPbX3, X=Cl, Br, and I): novel optoelectronic materials showing bright emission with wide color gamut. Nano Lett. 15, 3692–3696 (2015).
Folgueras, M. C. et al. Ligand-free processable perovskite semiconductor ink. Nano Lett. 21, 8856–8862 (2021).
Folgueras, M. C. et al. Lattice dynamics and optoelectronic properties of vacancy-ordered double perovskite Cs2TeX6 (X=Cl–, Br–, I–) single crystals. J. Phys. Chem. C 125, 25126–25139 (2021).
Blancon, J.-C. et al. Scaling law for excitons in 2D perovskite quantum wells. Nat. Commun. 9, 2254 (2018).
Barfüßer, A. et al. Confined excitons in spherical-like halide perovskite quantum dots. Nano Lett. 22, 8810–8817 (2022).
Baimuratov, A. S. & Högele, A. Valley-selective energy transfer between quantum dots in atomically thin semiconductors. Sci. Rep. 10, 16971 (2020).
Kovalev, D. et al. Resonant electronic energy transfer from excitons confined in silicon nanocrystals to oxygen molecules. Phys. Rev. Lett. 89, 137401 (2002).
Yeh, J.-W., Chen, Y.-L., Lin, S.-J. & Chen, S.-K. High-entropy alloys – a new era of exploitation. Mater. Sci. Forum 560, 1–9 (2007).
Zhang, R. et al. Short-range order and its impact on the CrCoNi medium-entropy alloy. Nature 581, 283–287 (2020).
Gali, A. & George, E. P. Tensile properties of high- and medium-entropy alloys. Intermetallics 39, 74–78 (2013).
Anand Sekhar, R., Samal, S., Nayan, N. & Bakshi, S. R. Microstructure and mechanical properties of Ti-Al-Ni-Co-Fe based high entropy alloys prepared by powder metallurgy route. J. Alloys Compd. 787, 123–132 (2019).
Solari, S. F. et al. Stabilization of lead-reduced metal halide perovskite nanocrystals by high-entropy alloying. J. Am. Chem. Soc. 144, 5864–5870 (2022).
Karim, M. M. S. et al. Anion distribution, structural distortion, and symmetry-driven optical band gap bowing in mixed halide Cs2SnX6 vacancy ordered double perovskites. Chem. Mater. 31, 9430–9444 (2019).
Hendrickson, W. A., Smith, J. L. & Sheriff, S. Direct phase determination based on anomalous scattering. Methods Enzymol. 115, 41–55 (1985).
Evans, G. & Pettifer, R. F. CHOOCH: a program for deriving anomalous-scattering factors from X-ray fluorescence spectra. J. Appl. Crystallogr. 34, 82–86 (2001).
Luo, H., Li, Z. & Raabe, D. Hydrogen enhances strength and ductility of an equiatomic high-entropy alloy. Sci. Rep. 7, 9892 (2017).
Kovalenko, M. V., Protesescu, L. & Bodnarchuk, M. I. Properties and potential optoelectronic applications of lead halide perovskite nanocrystals. Science 358, 745–750 (2017).
Zhong, Y. et al. Multi-dopant engineering in perovskite Cs2SnCl6: white light emitter and spatially luminescent heterostructure. Inorg. Chem. 60, 17357–17363 (2021).
Abfalterer, A. et al. Colloidal synthesis and optical properties of perovskite-inspired cesium zirconium halide nanocrystals. ACS Mater. Lett. 2, 1644–1652 (2020).
Saeki, K., Fujimoto, Y., Koshimizu, M., Yanagida, T. & Asai, K. Comparative study of scintillation properties of Cs2HfCl6 and Cs2ZrCl6. Appl. Phys. Express 9, 042602 (2016).
Wu, R., Liu, Y., Hu, S., Fu, P. & Xiao, Z. Red‐emitting perovskite variant Cs2PtCl6 phosphor: material design, luminous mechanism, and application in high‐color‐rendering white light‐emitting diodes. Adv. Opt. Mater. 10, 2201081 (2022).
McCall, K. M., Morad, V., Benin, B. M. & Kovalenko, M. V. Efficient lone-pair-driven luminescence: structure–property relationships in emissive 5s2 metal halides. ACS Mater. Lett. 2, 1218–1232 (2020).
Chang, T. et al. Efficient energy transfer in Te4+-doped Cs2ZrCl6 vacancy-ordered perovskites and ultrahigh moisture stability via A-site Rb-alloying strategy. J. Phys. Chem. Lett. 12, 1829–1837 (2021).
MacManus, J. P., Hogue, C. W., Marsden, B. J., Sikorska, M. & Szabo, A. G. Terbium luminescence in synthetic peptide loops from calcium-binding proteins with different energy donors. J. Biol. Chem. 265, 10358–10366 (1990).
Yun, C. S. et al. Nanometal surface energy transfer in optical rulers, breaking the FRET barrier. J. Am. Chem. Soc. 127, 3115–3119 (2005).
Zeltmann, A. H., Matwiyoff, N. A. & Morgan, L. O. Nuclear magnetic resonance of oxygen-17 and chlorine-35 in aqueous hydrochloric acid solutions of cobalt(II). I. Line shifts and relative abundances of solution species. J. Phys. Chem. 72, 121–127 (1968).
Brady, G. W., Robin, M. B. & Varimbi, J. The structure of ferric chloride in neutral and acid solutions. Inorg. Chem. 3, 1168–1173 (1964).
Tan, Z. et al. Lead‐free perovskite variant solid solutions Cs2Sn1–xTexCl6: bright luminescence and high anti‐water stability. Adv. Mater. 32, 2002443 (2020).
CrysAlisPro v.1.171.42.90a (Rigaku Corporation, 2022).
Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 42, 339–341 (2009).
Sheldrick, G. M. SHELXT – integrated space-group and crystal-structure determination. Acta Crystallogr. A Found. Adv. 71, 3–8 (2015).
Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 71, 3–8 (2015).
SAINT v.8.38A (Bruker AXS, Inc., 2012).
APEX3 v.2017.3-0 (Bruker AXS, Inc., 2012).
SADABS (Bruker AXS, Inc., 2017).
Gaussian v.16, Revision C.01, Frisch, M. J. et al. (Gaussian, Inc., 2016).
Gamelin, D. R. & Güdel, H. U. Spectroscopy and dynamics of Re4+ near-IR-to-visible luminescence upconversion. Inorg. Chem. 38, 5154–5164 (1999).
Khan, S. M., Patterson, H. H. & Engstrom, H. Multiple state luminescence for the d4 OsCl62– impurity ion in K2PtCl6 and Cs2ZrCl6 cubic crystals. Mol. Phys. 35, 1623–1636 (1978).
Douglas, I. N. Optical spectra of IrCl62− in single crystals of Cs2ZrCl6, Cs2HfCl6, and K2SnCl6 at low temperatures. J. Chem. Phys. 51, 3066–3073 (1969).
Heyd, J., Scuseria, G. E. & Ernzerhof, M. Hybrid functionals based on a screened Coulomb potential. J. Chem. Phys. 118, 8207–8215 (2003).
Andrae, D., Häußermann, U., Dolg, M., Stoll, H. & Preuß, H. Energy-adjusted ab initio pseudopotentials for the second and third row transition elements. Theor. Chim. Acta 77, 123–141 (1990).
Sousa, S. F. et al. Comparative analysis of the performance of commonly available density functionals in the determination of geometrical parameters for zinc complexes. J. Comput. Chem. 30, 2752–2763 (2009).
Lin, J. et al. Thermochromic halide perovskite solar cells. Nat. Mater. 17, 261–267 (2018).
Lin, Z., Folgueras, M. C., Le, H. K. D., Gao, M. & Yang, P. Laser-accelerated phase transformation in cesium lead iodide perovskite. Matter 5, 1455–1465 (2022).
Bischak, C. G. et al. Tunable polaron distortions control the extent of halide demixing in lead halide perovskites. J. Phys. Chem. Lett. 9, 3998–4005 (2018).
Yang, S. et al. Novel lead-free material Cs2PtI6 with narrow bandgap and ultra-stability for Its photovoltaic application. ACS Appl. Mater. Interfaces 12, 44700–44709 (2020).
Ju, M.-G. et al. Earth-abundant nontoxic titanium(IV)-based vacancy-ordered double perovskite halides with tunable 1.0 to 1.8 eV bandgaps for photovoltaic applications. ACS Energy Lett. 3, 297–304 (2018).
Sakai, N. et al. Solution-processed cesium hexabromopalladate(IV), Cs2PdBr6, for optoelectronic applications. J. Am. Chem. Soc. 139, 6030–6033 (2017).
Xu, Y. et al. Zero-dimensional Cs2TeI6 perovskite: solution-processed thick films with high X-ray sensitivity. ACS Photonics 6, 196–203 (2019).
Acknowledgements
This work was primarily supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences, Materials Sciences and Engineering Division, under contract no. DE-AC02-05CH11231 within the Fundamentals of Semiconductor Nanowire Program (KCPY23). The authors thank N. S. Settineri at UC Berkeley and S. J. Teat at the Advanced Light Source for their assistance in SCXRD and SCXRD–MAD measurements; A. J. Gubser for helpful discussions in regard to SEM–BSE imaging and EBSD measurements; and J. Grimsich for providing access to the various optical microscopes in the Earth and Planetary Sciences Petrographic Microscope Resource Center. Single-crystal X-ray diffraction studies were performed at the UC Berkeley College of Chemistry Small Molecule X-ray Crystallography Facility and at beamline 12.2.1 of the Advanced Light Source, a US DOE Office of Science User Facility at Lawrence Berkeley National Laboratory under contract no. DE-AC02-05CH11231. Ultra-low-frequency Raman spectroscopy was performed at the Stanford Nano Shared Facilities, supported by the National Science Foundation under award no. ECCS-2026822. M.C.F. acknowledges support from the Kavli ENSI Philomathia Graduate Student Fellowship. J.J. acknowledges fellowship support from Suzhou Industrial Park.
Author information
Authors and Affiliations
Contributions
M.C.F. and P.Y. conceived the idea. M.C.F., Y.J. and P.Y. designed the research. M.C.F. and Y.J. led the study and conducted materials synthesis, major characterization and data analysis including PXRD, SCXRD, SEM imaging, SEM–EDX, SEM–EBSD, ICP–AES, Raman, UV-visible absorption, photoluminescence and theoretical calculations. Y.J. and J.J. conducted SCXRD–MAD experiments. M.C.F. conducted X-ray fluorescence analysis. J.J. analysed SCXRD–MAD datasets. M.C.F., Y.J. and P.Y. wrote the manuscript. All authors discussed the results and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
This file contains Supplementary Sections 1–4 and a Supplementary Note, including Figs. 1–57 and Tables 1–19.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Folgueras, M.C., Jiang, Y., Jin, J. et al. High-entropy halide perovskite single crystals stabilized by mild chemistry. Nature 621, 282–288 (2023). https://doi.org/10.1038/s41586-023-06396-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-06396-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.