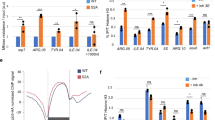
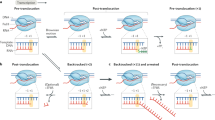
Abstract
The transcriptional machinery is thought to dissociate from DNA during replication. Certain proteins, termed epigenetic marks, must be transferred from parent to daughter DNA strands in order to maintain the memory of transcriptional states1,2. These proteins are believed to re-initiate rebuilding of chromatin structure, which ultimately recruits RNA polymerase II (Pol II) to the newly replicated daughter strands. It is believed that Pol II is recruited back to active genes only after chromatin is rebuilt3,4. However, there is little experimental evidence addressing the central questions of when and how Pol II is recruited back to the daughter strands and resumes transcription. Here we show that immediately after passage of the replication fork, Pol II in complex with other general transcription proteins and immature RNA re-associates with active genes on both leading and lagging strands of nascent DNA, and rapidly resumes transcription. This suggests that the transcriptionally active Pol II complex is retained in close proximity to DNA, with a Pol II–PCNA interaction potentially underlying this retention. These findings indicate that the Pol II machinery may not require epigenetic marks to be recruited to the newly synthesized DNA during the transition from DNA replication to resumption of transcription.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data supporting the findings of this study are available from the corresponding author on reasonable request.
References
Steffen, P. A. & Ringrose, L. What are memories made of? How Polycomb and Trithorax proteins mediate epigenetic memory. Nat. Rev. Mol. Cell Biol. 15, 340–356 (2014).
Bertoli, C., Skotheim, J. M. & de Bruin, R. A. M. Control of cell cycle transcription during G1 and S phases. Nat. Rev. Mol. Cell Biol. 14, 518–528 (2013).
Stewart-Morgan, K. R., Petryk, N. & Groth, A. Chromatin replication and epigenetic cell memory. Nat. Cell Biol. 22, 361–371 (2020).
Almouzni, G. & Cedar, H. Maintenance of epigenetic information. Cold Spring Harb. Perspect. Biol. 8, a019372 (2016).
Li, B., Carey, M. & Workman, J. L. The role of chromatin during transcription. Cell 128, 707–719 (2007).
Kadauke, S. & Blobel, G. A. Mitotic bookmarking by transcription factors. Epigenetics Chromatin 6, 6 (2013).
Petruk, S. et al. TrxG and PcG proteins but not methylated histones remain associated with DNA through replication. Cell 150, 922–933 (2012).
Petruk, S., Black, K. L., Kovermann, S. K., Brock, H. W. & Mazo, A. Stepwise histone modifications are mediated by multiple enzymes that rapidly associate with nascent DNA during replication. Nat. Commun. 4, 2841 (2013).
Herrick, J. & Bensimon, A. Global regulation of genome duplication in eukaryotes: an overview from the epifluorescence microscope. Chromosoma 117, 243–260 (2008).
Buratowski, S. Progression through the RNA polymerase II CTD cycle. Mol. Cell 36, 541–546 (2009).
Kwiatkowski, N. et al. Targeting transcription regulation in cancer with a covalent CDK7 inhibitor. Nature 511, 616–620 (2014).
Titov, D. V. et al. XPB, a subunit of TFIIH, is a target of the natural product triptolide. Nat. Chem. Biol. 7, 182–188 (2011).
Wada, T. et al. DSIF, a novel transcription elongation factor that regulates RNA polymerase II processivity, is composed of human Spt4 and Spt5 homologs. Genes Dev. 12, 343–356 (1998).
Mancebo, H. S. Y. et al. P-TEFb kinase is required for HIV Tat transcriptional activation in vivo and in vitro. Genes Dev. 11, 2633–2644 (1997).
Adelman, K. & Lis, J. T. Promoter-proximal pausing of RNA polymerase II: emerging roles in metazoans. Nat. Rev. Genet. 13, 720–731 (2012).
Shandilya, J. & Roberts, S. G. E. The transcription cycle in eukaryotes: From productive initiation to RNA polymerase II recycling. Biochim. Biophys. Acta - Gene Regul. Mech. 1819, 391–400 (2012).
Yudkovsky, N., Ranish, J. A. & Hahn, S. A transcription reinitiation intermediate that is stabilized by activator. Nature 408, 225–229 (2000).
Jonkers, I. & Lis, J. T. Getting up to speed with transcription elongation by RNA polymerase II. Nat. Rev. Mol. Cell Biol. 16, 167–177 (2015).
Leuchowius, K.-J. et al. Parallel visualization of multiple protein complexes in individual cells in tumor tissue. Mol. Cell. Proteomics 12, 1563–1571 (2013).
McKnight, S. L. & Miller, O. L. Electron microscopic analysis of chromatin replication in the cellular blastoderm Drosophila melanogaster embryo. Cell 12, 795–804 (1977).
Franklin, R. E. & Gosling, R. G. Molecular configuration in sodium thymonucleate. Nature 171, 740–741 (1953).
Burgers, P. M. J. & Kunkel, T. A. Eukaryotic DNA replication fork. Annu. Rev. Biochem. 86, 417–438 (2017).
Francis, N. J., Follmer, N. E., Simon, M. D., Aghia, G. & Butler, J. D. Polycomb proteins remain bound to chromatin and DNA during DNA replication in vitro. Cell 137, 110–122 (2009).
Petruk, S., Fenstermaker, T. K., Black, K. L., Brock, H. W. & Mazo, A. Detection of RNA–DNA association by a proximity ligation-based method. Sci. Rep. 6, 27313 (2016).
Fenstermaker, T. K., Sun, G., Mazo, A. & Petruk, S. A proximity ligation-based method to detect RNA–DNA association. Methods Mol. Biol. 2008, 121–129 (2019).
Geiger, F. et al. Liquid–liquid phase separation underpins the formation of replication factories in rotaviruses. EMBO J. 40, e107711 (2021).
Parker, M. W., Kao, J. A., Huang, A., Berger, J. M. & Botchan, M. R. Molecular determinants of phase separation for Drosophila DNA replication licensing factors. eLife 10, e70535 (2021).
Lai, J. S. & Herr, W. Ethidium bromide provides a simple tool for identifying genuine DNA-independent protein associations. Proc. Natl Acad. Sci. USA 89, 6958–6962 (1992).
Moldovan, G.-L. L., Pfander, B. & Jentsch, S. PCNA, the maestro of the replication fork. Cell 129, 665–679 (2007).
González-Magaña, A. & Blanco, F. J. Human PCNA structure, function and interactions. Biomolecules 10, 570 (2020).
Tan, Z. et al. Small-molecule targeting of proliferating cell nuclear antigen chromatin association inhibits tumor cell growth. Mol. Pharmacol. 81, 811–819 (2012).
Ito, K. & Zaret, K. S. Maintaining transcriptional specificity through mitosis. Annu. Rev. Genomics Hum. Genet. 23, 53–71 (2022).
Bellec, M. et al. The control of transcriptional memory by stable mitotic bookmarking. Nat. Commun. 13, 1176 (2022).
Liu, B. & Alberts, B. M. Head-on collision between a DNA replication apparatus and RNA polymerase transcription complex. Science 267, 1131–1137 (1995).
Liu, B., Wong, M. L., Tinker, R. L., Geiduschek, E. P. & Alberts, B. M. The DNA replication fork can pass RNA polymerase without displacing the nascent transcript. Nature 366, 33–39 (1993).
Pomerantz, R. T. & O’Donnell, M. What happens when replication and transcription complexes collide? Cell Cycle 9, 2537–2543 (2010).
Leung, K. H. T., Abou El Hassan, M. & Bremner, R. A rapid and efficient method to purify proteins at replication forks under native conditions. Biotechniques 55, 204–206 (2013).
Stewart-Morgan, K. R., Reverón-Gómez, N. & Groth, A. Transcription restart establishes chromatin accessibility after DNA replication. Mol. Cell 75, 284–297.e6 (2019).
Hassan, A. B., Errington, R. J., White, N. S., Jackson, D. A. & Cook, P. R. Replication and transcription sites are colocalized in human cells. J. Cell Sci. 107, 425–434 (1994).
Malyavantham, K. S., Bhattacharya, S. & Berezney, R. The architecture of functional neighborhoods within the mammalian cell nucleus. Adv. Enzyme Regul. 50, 126–134 (2010).
Wansink, D. G. et al. RNA polymerase II transcription is concentrated outside replication domains throughout S-phase. J. Cell Sci. 107, 1449–1456 (1994).
Wei, X. et al. Segregation of transcription and replication sites into higher order domains. Science 281, 1502–1506 (1998).
Helmrich, A., Ballarino, M. & Tora, L. Collisions between replication and transcription complexes cause common fragile site instability at the longest human genes. Mol. Cell 44, 966–977 (2011).
Rimel, J. K. & Taatjes, D. J. The essential and multifunctional TFIIH complex. Protein Sci. 27, 1018–1037 (2018).
Alzu, A. et al. Senataxin associates with replication forks to protect fork integrity across RNA-polymerase-II-transcribed genes. Cell 151, 835–846 (2012).
Groh, M., Albulescu, L. O., Cristini, A. & Gromak, N. Senataxin: genome guardian at the interface of transcription and neurodegeneration. J. Mol. Biol. 429, 3181–3195 (2017).
Hasanova, Z., Klapstova, V., Porrua, O., Stefl, R. & Sebesta, M. Human senataxin is a bona fide R-loop resolving enzyme and transcription termination factor. Nucleic Acids Res. 51, 2818–2837 (2023).
Andrs, M., Hasanova, Z., Oravetzova, A., Dobrovolna, J. & Janscak, P. RECQ5: A mysterious helicase at the interface of DNA replication and transcription. Genes 11, 232 (2020).
Wang, J. et al. Persistence of RNA transcription during DNA replication delays duplication of transcription start sites until G2/M. Cell Rep. 34, 108759 (2021).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 (2012).
McCloy, R. A. et al. Partial inhibition of Cdk1 in G2 phase overrides the SAC and decouples mitotic events. Cell Cycle 13, 1400–1412 (2014).
Acknowledgements
The authors thank B. Calabretta and S. McMahon for feedback on experiments and the manuscript. This work was supported by the following grants: NIH R01GM075141 to A.M.; T.K.F. was supported by NIH F31GM128300 and NIH training grant T32GM100836. Leica SP8 STED microscope imaging was supported by the Bioimaging Shared Resource of the Sidney Kimmel Cancer Center (NCI 5 P30 CA-56036).
Author information
Authors and Affiliations
Contributions
T.K.F., S.P., H.W.B. and A.M. conceptualized the study. T.K.F. performed experiments presented in Figs. 1–5 and Extended Data Figs. 1–4, 6 and 7. S.P. performed experiments in Extended Data Fig. 5 and performed preliminary experiments pertaining to the results in Figs. 1 and 5 and Extended Data Fig. 4. S.K.K performed preliminary experiments pertaining to the results in Fig. 1. T.K.F. and S.P. designed and developed the RDIA assay. T.K.F. developed the multiplex CAA assay. T.K.F. wrote the manuscript with input from S.P., H.W.B. and A.M. S.P., H.W.B., T.K.F. and A.M. acquired the funding. A.M. provided overall supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Martijn Luijsterburg, Sheila Teves and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 CAA reactions with IgG control do not generate PLA signals.
Cells were labelled for 10 min and PLA was performed using the indicated antibodies and an IgG control. Red, PLA, Green, EdU, Blue, DAPI. Scale bar = 10 μm. Data shown are representative of 3 independent experiments.
Extended Data Fig. 2 Detection of RNA Pol II on nascent DNA is not the result of a new round of transcription.
a, Cells were treated for 30 min 2 h, or 4 h with the indicated inhibitors or left untreated as a control and then labelled with EdU for 10 min in the presence of the inhibitors and CAA was performed for Pol II S5P (left) or Pol II S2P (right). Data are presented as mean values ± s.d. (n = 3 independent experiments). For each of the following, the number of nuclei examined over 3 independent experiments are: 112 (S5P top), 153 (S5P bottom), 70 (S2P top), and 139 (S2P bottom). Statistical significance was determined by one-way ANOVA and p-values shown were determined with Tukey’s post-hoc. ns = not significant, **P < 0.01, ****P < 0.0001. b, Cells were treated for 30 min with either THZ-1 or DRB or left untreated as a control and then blotted for the indicated proteins. Data shown are representative of 3 independent experiments. For blot source data, see Supplementary Fig. 1. c, Cells were treated with either TPL or DRB or left untreated as a control and then labelled with EdU for 10 min in the presence of the inhibitors, and CAA was performed for PCNA. Data are presented as mean values ± s.d. (n = 3 independent experiments), and 117 nuclei were examined over 3 independent experiments, and statistical significance was determined by one-way ANOVA. ns = not significant. d, Cells were treated for 4 h with DRB or left untreated as a control and then labelled with EdU for 10 min in the presence of the inhibitors and CAA was performed for TBP. Data are presented as mean values ± s.d. (n = 3 independent experiments), 77 nuclei were examined over 3 independent experiments, and statistical significance was determined by two-way, unpaired t-test. ns = not significant. e, Cells were treated for 4 h with DRB or left untreated as a control and then labelled with EdU for 10 min in the presence of the inhibitors and CAA was performed for either SPT4 (top) or SPT5 (bottom). Data are presented as mean values ± s.d. (n = 3 independent experiments), and 77 (SPT4) or 88 (SPT5) nuclei were examined over 3 independent experiments. Statistical significance was determined by two-way, unpaired t-test. ****P < 0.0001.
Extended Data Fig. 3 Multiplex CAA detects proteins close to each other on labelled DNA.
a, Schematic representation of multiplex CAA illustrating that while 7.4 to 12 kb of DNA is labeled in 10 min, overlapping PLA signals are on average within 400 bp of one another. b, Quantification of the percent of overlaps following 10 min and 20 min EdU labelling for multiplex CAA with PCNA and either Pol II S5P (top) or Pol II S2P (bottom). Data are presented as mean values ± s.d. from n = 5 independent samples. Statistical significance was determined by a two-way, unpaired t-test. **P < 0.01, ****P < 0.0001. c, Direct PLA between replication proteins: Pol ε and PCNA, Pol ε and FEN1, and FEN1 and PCNA. Data are presented as mean values ± s.d. (n = 3 independent experiments), and 152 nuclei were examined over 3 independent experiments. Statistical significance was determined by one-way ANOVA and p-values shown were determined with Tukey’s post-hoc. ****P < 0.0001.
Extended Data Fig. 4 RNA polymerase II associates with target genes on nascent DNA.
a, Schematic representation of BrdU re-ChIP. DNA was labelled with BrdU (red circle) and then immunoprecipitated with anti-Pol II antibody. Following reverse crosslinking, DNA was denatured and immunoprecipitated with anti-BrdU antibody. Recovered DNA was analyzed by qPCR. Red ring is PCNA, replisome is in blue. b, Schematic representing locations of qPCR products within each analysed gene. Bent arrow represents the TSS and pA indicates the polyadenylation site. For each gene two sets of primers were designed: one close to the TSS and one farther downstream in the gene. c, Pol II ChIP. ChIP was performed for Pol II or IgG and subsequently qPCR was performed at upstream and downstream regions the active genes GAPDH, PPIA, and TNFAIP3 and at the repressed gene GFAP. d, BrdU re-ChIP following 25 min BrdU labelling. DNA was labelled with BrdU for 25 min or unlabelled as a control. ChIP was performed for Pol II or IgG. Following purification of DNA, Pol II ChIP samples were subjected to a second round of immunoprecipitation for BrdU. e, BrdU re-ChIP following 40 min BrdU labelling. DNA was labelled with BrdU for 40 min or unlabelled as a control. ChIP was performed for Pol II or IgG. Following purification of DNA, Pol II ChIP samples were subjected to a second round of immunoprecipitation for BrdU. Numbering represents primer sets shown in b. Data in c–e are presented as mean values and are representative of 2 independent experiments.
Extended Data Fig. 5 PCNA immunoprecipitates short fragments of nascent DNA.
a, Top: Primer map showing forward primer (at position zero) and reverse primers at increasing distances within the TNFAIP3 gene. Bottom: The percent of input for PCNA immunoprecipitated DNA either without S1 nuclease (left) or following digestion with 1 U S1 nuclease (right). b, Top: Primer map showing forward primer (at position zero) and reverse primers at increasing distances within the GFAP gene. Bottom: The percent of input for PCNA immunoprecipitated DNA either without S1 nuclease (left) or following digestion with 1 U S1 nuclease (right). c, m13mp18 ssDNA was treated with 1 U S1 nuclease for 10 min in order to ensure that single stranded DNA was completely digested under treatment conditions. Data in a–c are presented as mean values and are representative of 2 independent experiments.
Extended Data Fig. 6 PCNA is a mark of nascent chromatin throughout the genome.
a, PCNA ChIP. Cells were immunoprecipitated with an anti-PCNA antibody or with IgG and analysed by qPCR at upstream and downstream regions the active genes GAPDH, PPIA, and TNFAIP3 and at the repressed gene GFAP. Numbering represents primer sets shown in Extended Data Fig. 4b. Data are presented as mean values and are representative of 2 independent experiments. b, Cells were labelled for 5 min with EdU, chased for the indicated times, and then CAA was performed for PCNA. Data are presented as mean values ± s.d. (n = 3 independent experiments), and 208 nuclei were examined over 3 independent experiments. Statistical significance was determined by one-way ANOVA and p-values shown were determined with Tukey’s post-hoc. ns = not significant, ****P < 0.0001. c, Sequential ChIP with PCNA and SPT4. Upper panel: ChIP was performed for SPT4 or IgG and then assessed by PCR at the same genes indicated in Extended Data Fig. 4b. Lower panel: re-ChIP of samples first immunoprecipitated for SPT4. Following elution, samples were re-immunoprecipitated for PCNA or IgG. Following DNA purification, samples were analyzed by qPCR. Data are presented as mean values and are representative of 2 independent experiments. d, Western blotting validation of PCNA and FEN1 polyclonal antibodies generated in this study. Data shown are representative of 3 independent experiments. For blot source data, see Supplementary Fig. 1.
Extended Data Fig. 7 Pol II transfer to nascent DNA is likely not the result of LLPS and the interaction between PCNA and Pol II is DNA-independent.
a, Cells were labelled for 30 min with BrU and then labelled for 10 min with EdU in the presence of either THZ-1 or DRB or left untreated as a control. Subsequently, RDIA was performed to assess immature RNA retention post-replication. Data are presented as mean values ± s.d. (n = 3 independent experiments), and 84 nuclei were examined over 3 independent experiments. Statistical significance was determined by one-way ANOVA. ns = not significant. b, Cells were labelled for 15 min with EdU either in the presence of 2% hexanediol, 3% hexanediol, or without hexanediol as a control, then CAA was performed for Pol II S5P (top) or Pol II S2P (bottom). Data are presented as mean values ± s.d. (n = 3 independent experiments). 50 nuclei were examined over 3 independent experiments. Statistical significance was determined by one-way ANOVA. ns = not significant. c, Nuclear extracts were either treated or untreated with ethidium bromide prior to immunoprecipitation with anti-PCNA antibody and subsequent western blotting. Data shown are representative of 3 independent experiments. For blot source data, see Supplementary Fig. 1. d, Top: Cells were untreated or treated with 3 µM PCNA-I1 for the indicated times and then EdU labelled and fixed. Bottom: Cells were untreated or treated with 3 µM PCNA-I1 for 3 h and then the inhibitor was washed out for the indicated times. Cells were labelled with EdU and fixed. In both experiments, following fixation, biotin was conjugated to EdU using click chemistry and immunostained in order to assess DNA replication. Corrected cell total fluorescence (CTCF) was quantified as described in the methods. Data are presented as mean values ± s.d. (n = 3 independent experiments), and 140 (top) or 94 (bottom) nuclei were examined over 3 independent experiments. Statistical significance was determined by one-way ANOVA and p-values shown were determined by Tukey’s post-hoc. ns = not significant, *P < 0.05, **P < 0.01, ****P < 0.0001.
Supplementary information
Supplementary Information
This file contains Supplemental Table 1: List of PCR primers used in this study; and Supplementary Fig. 1: Source western blot images.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fenstermaker, T.K., Petruk, S., Kovermann, S.K. et al. RNA polymerase II associates with active genes during DNA replication. Nature 620, 426–433 (2023). https://doi.org/10.1038/s41586-023-06341-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-06341-9
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.