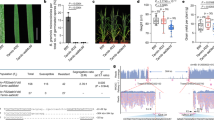
Abstract
The discovery and application of genome editing introduced a new era of plant breeding by giving researchers efficient tools for the precise engineering of crop genomes1. Here we demonstrate the power of genome editing for engineering broad-spectrum disease resistance in rice (Oryza sativa). We first isolated a lesion mimic mutant (LMM) from a mutagenized rice population. We then demonstrated that a 29-base-pair deletion in a gene we named RESISTANCE TO BLAST1 (RBL1) caused broad-spectrum disease resistance and showed that this mutation caused an approximately 20-fold reduction in yield. RBL1 encodes a cytidine diphosphate diacylglycerol synthase that is required for phospholipid biosynthesis2. Mutation of RBL1 results in reduced levels of phosphatidylinositol and its derivative phosphatidylinositol 4,5-bisphosphate (PtdIns(4,5)P2). In rice, PtdIns(4,5)P2 is enriched in cellular structures that are specifically associated with effector secretion and fungal infection, suggesting that it has a role as a disease-susceptibility factor3. By using targeted genome editing, we obtained an allele of RBL1, named RBL1Δ12, which confers broad-spectrum disease resistance but does not decrease yield in a model rice variety, as assessed in small-scale field trials. Our study has demonstrated the benefits of editing an LMM gene, a strategy relevant to diverse LMM genes and crops.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Sequencing data for the pooled M3 plants derived from the rbl1 line (FN398) are available at the National Center for Biotechnology Information under the accession number SRR4096918. Other sequences can be accessed under the following numbers: RBL1 (LOC_Os01g55360, NP_001044302.1), OsPIS1 (LOC_Os02g03110, NC_029257.1) and OsPAH2 (LOC_Os11g40080, XP_015617116.1). Original data points in graphs are shown in the source data files. Uncropped gel and immunoblotting images can be found in Supplementary Fig. 1. All plasmids and plant lines generated in this work are available from the authors upon request. Source data are provided with this paper.
References
Gao, C. Genome engineering for crop improvement and future agriculture. Cell 184, 1621–1635 (2021).
Liu, X., Yin, Y., Wu, J. & Liu, Z. Structure and mechanism of an intramembrane liponucleotide synthetase central for phospholipid biosynthesis. Nat. Commun. 5, 4244 (2014).
Qin, L. et al. Specific recruitment of phosphoinositide species to the plant–pathogen interfacial membrane underlies Arabidopsis susceptibility to fungal infection. Plant Cell 32, 1665–1688 (2020).
Zhu, H., Li, C. & Gao, C. Applications of CRISPR–Cas in agriculture and plant biotechnology. Nat. Rev. Mol. Cell Biol. 21, 661–677 (2020).
Chen, K., Wang, Y., Zhang, R., Zhang, H. & Gao, C. CRISPR/Cas genome editing and precision plant breeding in agriculture. Annu. Rev. Plant Biol. 70, 667–697 (2019).
Sakulkoo, W. et al. A single fungal MAP kinase controls plant cell-to-cell invasion by the rice blast fungus. Science 359, 1399–1403 (2018).
Li, W., Chern, M., Yin, J., Wang, J. & Chen, X. Recent advances in broad-spectrum resistance to the rice blast disease. Curr. Opin. Plant Biol. 50, 114–120 (2019).
Song, W.-Y. et al. A receptor kinase-like protein encoded by the rice disease resistance gene, Xa21. Science 270, 1804–1806 (1995).
Li, W. et al. A natural allele of a transcription factor in rice confers broad-spectrum blast resistance. Cell 170, 114–126 (2017).
Deng, Y. et al. Epigenetic regulation of antagonistic receptors confers rice blast resistance with yield balance. Science 355, 962–965 (2017).
Wang, J. et al. A single transcription factor promotes both yield and immunity in rice. Science 361, 1026–1028 (2018).
Gao, M. et al. Ca2+ sensor-mediated ROS scavenging suppresses rice immunity and is exploited by a fungal effector. Cell 184, 5391–5404 (2021).
Hu, X.-H. et al. A natural allele of proteasome maturation factor improves rice resistance to multiple pathogens. Nat. Plants 9, 228–237 (2023).
Krattinger, S. G. et al. A putative ABC transporter confers durable resistance to multiple fungal pathogens in wheat. Science 323, 1360–1363 (2009).
Wang, N. et al. Inactivation of a wheat protein kinase gene confers broad-spectrum resistance to rust fungi. Cell 185, 2961–2974 (2022).
Büschges, R. et al. The barley Mlo gene: a novel control element of plant pathogen resistance. Cell 88, 695–705 (1997).
Li, S. et al. Genome-edited powdery mildew resistance in wheat without growth penalties. Nature 602, 455–460 (2022).
Lorrain, S., Vailleau, F., Balagué, C. & Roby, D. Lesion mimic mutants: keys for deciphering cell death and defense pathways in plants?. Trends Plant Sci. 8, 263–271 (2003).
Xing, J., Zhang, L., Duan, Z. & Lin, J. Coordination of phospholipid-based signaling and membrane trafficking in plant immunity. Trends Plant Sci. 26, 407–420 (2021).
Liu, L., Waters, D. L. E., Rose, T. J., Bao, J. & King, G. J. Phospholipids in rice: significance in grain quality and health benefits: a review. Food Chem. 139, 1133–1145 (2013).
Shimada, T. L. et al. Enrichment of phosphatidylinositol 4,5-bisphosphate in the extra-invasive hyphal membrane promotes colletotrichum infection of Arabidopsis thaliana. Plant Cell Physiol. 60, 1514–1524 (2019).
Li, G. et al. The sequences of 1504 mutants in the model rice variety Kitaake facilitate rapid functional genomic studies. Plant Cell 29, 1218–1231 (2017).
You, Q. et al. An E3 ubiquitin ligase-BAG protein module controls plant innate immunity and broad-spectrum disease resistance. Cell Host Microbe 20, 758–769 (2016).
Zhao, X. et al. A novel glycine-rich domain protein, GRDP1, functions as a critical feedback regulator for controlling cell death and disease resistance in rice. J. Exp. Bot. 72, 608–622 (2021).
Liu, Q. et al. OsCUL3a negatively regulates cell death and immunity by degrading OsNPR1 in rice. Plant Cell 29, 345–359 (2017).
Zhou, Y. et al. Extraplastidial cytidinediphosphate diacylglycerol synthase activity is required for vegetative development in Arabidopsis thaliana. Plant J. 75, 867–879 (2013).
Blunsom, N. J. & Cockcroft, S. CDP-diacylglycerol synthases (CDS): gateway to phosphatidylinositol and cardiolipin synthesis. Front. Cell Dev. Biol. 8, 63 (2020).
Li, J. & Wang, X. Phospholipase D and phosphatidic acid in plant immunity. Plant Sci. 279, 45–50 (2019).
Khang, C. H. et al. Translocation of Magnaporthe oryzae effectors into rice cells and their subsequent cell-to-cell movement. Plant Cell 22, 1388–1403 (2010).
Du, X. Q., Yao, H. Y., Luo, P., Tang, X. C. & Xue, H. W. Cytidinediphosphate diacylglycerol synthase—mediated phosphatidic acid metabolism is crucial for early embryonic development of Arabidopsis. PLoS Genet. 18, e1010320 (2022).
Hong, Y., Yuan, S., Sun, L., Wang, X. & Hong, Y. Cytidinediphosphate-diacylglycerol synthase 5 is required for phospholipid homeostasis and is negatively involved in hyperosmotic stress tolerance. Plant J. 94, 1038–1050 (2018).
Di Paolo, G. & De Camilli, P. Phosphoinositides in cell regulation and membrane dynamics. Nature 443, 651–657 (2006).
Kale, S. D. et al. External lipid PI3P mediates entry of eukaryotic pathogen effectors into plant and animal host cells. Cell 142, 284–295 (2010); erratum 142, 981–983 (2010).
Liu, L. et al. The Xanthomonas type III effector XopAP prevents stomatal closure by interfering with vacuolar acidification. J. Integr. Plant Biol. 64, 1994–2008 (2022).
Wang, H. et al. A plant virus hijacks phosphatidylinositol-3,5-bisphosphate to escape autophagic degradation in its insect vector. Autophagy 19, 1128–1143 (2023).
Marković, V. & Jaillais, Y. Phosphatidylinositol 4-phosphate: a key determinant of plasma membrane identity and function in plants. New Phytol. 235, 867–874 (2022).
Boni, R. et al. Pathogen-inducible Ta-Lr34res expression in heterologous barley confers disease resistance without negative pleiotropic effects. Plant Biotechnol. J. 16, 245–253 (2018).
Gruner, K. et al. Evidence for allele-specific levels of enhanced susceptibility of wheat mlo mutants to the hemibiotrophic fungal pathogen Magnaporthe oryzae pv. Triticum. Genes 11, 517 (2020).
Li, W., Deng, Y., Ning, Y., He, Z. & Wang, G.-L. Exploiting broad-spectrum disease resistance in crops: from molecular dissection to breeding. Annu. Rev. Plant Biol. 71, 575–603 (2020).
Oliva, R. et al. Broad-spectrum resistance to bacterial blight in rice using genome editing. Nat. Biotechnol. 37, 1344–1350 (2019).
Lu, Y. et al. Targeted, efficient sequence insertion and replacement in rice. Nat. Biotechnol. 38, 1402–1407 (2020).
Li, G. et al. Genome-wide sequencing of 41 rice (Oryza sativa L.) mutated lines reveals diverse mutations induced by fast-neutron irradiation. Mol. Plant 9, 1078–1081 (2016).
Jain, R. et al. Genome sequence of the model rice variety KitaakeX. BMC Genom. 20, 905 (2019).
Tang, J. et al. GDSL lipase occluded stomatal pore 1 is required for wax biosynthesis and stomatal cuticular ledge formation. New Phytol. 228, 1880–1896 (2020).
Park, C.-J. & Ronald, P. C. Cleavage and nuclear localization of the rice XA21 immune receptor. Nat. Commun. 3, 920 (2012).
Zhou, X. et al. Loss of function of a rice TPR-domain RNA-binding protein confers broad-spectrum disease resistance. Proc. Natl Acad. Sci. USA 115, 3174–3179 (2018).
Li, G. et al. MST50 is involved in multiple MAP kinase signaling pathways in Magnaporthe oryzae. Environ. Microbiol. 19, 1959–1974 (2017).
Li, G. B. et al. Overproduction of OsRACK1A, an effector-targeted scaffold protein promoting OsRBOHB-mediated ROS production, confers rice floral resistance to false smut disease without yield penalty. Mol. Plant 15, 1790–1806 (2022).
Li, Q. et al. A Phytophthora capsici effector targets ACD11 binding partners that regulate ROS-mediated defense response in Arabidopsis. Mol. Plant 12, 565–581 (2019).
DeZwaan, T. M., Carroll, A. M., Valent, B. & Sweigard, J. A. Magnaporthe grisea Pth11p is a novel plasma membrane protein that mediates appressorium differentiation in response to inductive substrate cues. Plant Cell 11, 2013–2030 (1999).
Jones, K. et al. Disruption of the interfacial membrane leads to Magnaporthe oryzae effector re-location and lifestyle switch during rice blast disease. Front. Cell Dev. Biol. 9, 681734 (2021).
Chen, X. et al. An ATPase promotes autophosphorylation of the pattern recognition receptor XA21 and inhibits XA21-mediated immunity. Proc. Natl Acad. Sci. USA 107, 8029–8034 (2010).
Luu, D. D. et al. Biosynthesis and secretion of the microbial sulfated peptide RaxX and binding to the rice XA21 immune receptor. Proc. Natl Acad. Sci. USA 116, 8525–8534 (2019).
Fan, J. et al. The monocot-specific receptor-like kinase SDS2 controls cell death and immunity in rice. Cell Host Microbe 23, 498–510 (2018).
Chen, X. et al. The ‘pears and lemons’ protein UvPal1 regulates development and virulence of Ustilaginoidea virens. Environ. Microbiol. 22, 5414–5432 (2020).
Fan, J. et al. The false smut pathogen Ustilaginoidea virens requires rice stamens for false smut ball formation. Environ. Microbiol. 22, 646–659 (2020).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the \({2}^{-\Delta \Delta {C}_{{\rm{T}}}}\) method. Methods 25, 402–408 (2001).
Haselier, A., Akbari, H., Weth, A., Baumgartner, W. & Frentzen, M. Two closely related genes of Arabidopsis encode plastidial cytidinediphosphate diacylglycerol synthases essential for photoautotrophic growth. Plant Physiol. 153, 1372–1384 (2010).
Durowoju, I. B., Bhandal, K. S, Hu, J., Carpick, B. & Kirkitadze, M. Differential scanning calorimetry — a method for assessing the thermal stability and conformation of protein antigen. J. Vis. Exp. 121, e55262 (2017).
Li, Q. et al. Understanding the biochemical basis of temperature-induced lipid pathway adjustments in plants. Plant Cell 27, 86–103 (2015).
Lu, S., Liu, H., Jin, C., Li, Q. & Guo, L. An efficient and comprehensive plant glycerolipids analysis approach based on high-performance liquid chromatography-quadrupole time-of-flight mass spectrometer. Plant Direct 3, e00183 (2019).
Ito, Y. et al. Sphingolipids mediate polar sorting of PIN2 through phosphoinositide consumption at the trans-Golgi network. Nat. Commun. 12, 4267 (2021).
He, F., Zhang, F., Sun, W., Ning, Y. & Wang, G.-L. A versatile vector toolkit for functional analysis of rice genes. Rice 11, 27 (2018).
Santoni, V. Plant plasma membrane protein extraction and solubilization for proteomic analysis. Methods Mol. Biol. 355, 93–109 (2007).
Yang, H. et al. Pore architecture of TRIC channels and insights into their gating mechanism. Nature 538, 537–541 (2016).
Colin, L. et al. Imaging the living plant cell: from probes to quantification. Plant Cell 34, 247–272 (2022).
Tang, N. et al. MODD mediates deactivation and degradation of OsbZIP46 to negatively regulate ABA signaling and drought resistance in rice. Plant Cell 28, 2161–2177 (2016).
Zhai, K. et al. NLRs guard metabolism to coordinate pattern- and effector-triggered immunity. Nature 601, 245–251 (2022).
Gutteridge, S. & Gatenby, A. A. Rubisco synthesis, assembly, mechanism, and regulation. Plant Cell 7, 809–819 (1995).
Simon, M. L. et al. A multi-colour/multi-affinity marker set to visualize phosphoinositide dynamics in Arabidopsis. Plant J. 77, 322–337 (2014).
Xie, K., Minkenberg, B. & Yang, Y. Boosting CRISPR/Cas9 multiplex editing capability with the endogenous tRNA-processing system. Proc. Natl Acad. Sci. USA 112, 3570–3575 (2015).
Minkenberg, B., Zhang, J., Xie, K. & Yang, Y. CRISPR-PLANT v2: an online resource for highly specific guide RNA spacers based on improved off-target analysis. Plant Biotechnol. J. 17, 5–8 (2019).
Xie, X. et al. CRISPR-GE: a convenient software toolkit for CRISPR-based genome editing. Mol. Plant 10, 1246–1249 (2017).
IRRI. Standard Evaluation System for Rice (SES) 17–18 (International Rice Research Institute, 2002).
Yang, L. et al. The genome of the rice variety LTH provides insight into its universal susceptibility mechanism to worldwide rice blast fungal strains. Comput. Struct. Biotechnol. J. 20, 1012–1026 (2022).
Acknowledgements
We thank G. Wang and Y. Ning for the pRGV and pRHV vectors; X. Chen and D. Dou for the M. oryzae and P. capsici strains, respectively; Z. Wang for the Pwl2–mCherry marker; W. Zhao and L. Cao for the rice spl-D and cul3a mutants, respectively; H. Xue for the Arabidopsis cds mutants; P. Fei for technical support with confocal imaging; and G. Ren for the field trials; H. Hu for the pEarleyGate101 vector and HDEL–mCherry markers; D. Duanmu for the pYES2–NTA vectors; W. Chen for the total salicylic acid analysis; C. Luo, M. Yuan and Z. Lai for the U. virens, Xoo strains and Arabidopsis lines; Z. Hu and X. Shen for their support with data acquisition and analysis; and J. Zhou, X. Chen, H. He, X. Wang and J. R. Xu for discussions. The phospholipid analysis was performed on the lipidomics platform of the National Key Laboratory of Crop Genetic Improvement. Confocal laser scanning microscope data were acquired at the National Key Laboratory of Agricultural Microbiology Core Facility. The computations in this paper were run on the bioinformatics computing platform of the National Key Laboratory of Crop Genetic Improvement. This work was supported by the National Key R&D Program of China (2022YFA1304402), Natural Science Foundation of China (32172373 and 31801723), Fundamental Research Funds for the Central Universities (2662020ZKPY006, 2023ZKPY002 and 2662023PY006) and the Open Research Fund of the State Key Laboratory of Hybrid Rice (Wuhan University) (KF202202) to G.L. J.C.M. is supported by the Joint Bioenergy Institute funded by the US Department of Energy (DE-AC02-05CH11231). The phospholipid measurements were performed with support from the Bordeaux Metabolome Facility-MetaboHUB (ANR-11-INBS-0010) and Fundamental Research Funds for the Central Universities (2662020ZKPY005). Research in the Ronald Laboratory was supported by the National Science Foundation, the National Institutes of Health (GM122968 and GM55962) and the Joint Bioenergy Institute funded by the US Department of Energy (DE-AC02-05CH11231).
Author information
Authors and Affiliations
Contributions
G.L., G.S., P.S. and P.C.R. designed the experiments. G.L. and R.J. screened and analysed the genomic data of the rbl1 mutant. G.S., P.S., X.K., X.H., Y.L., Y.W., Q.G., X.C. and L.Z. performed plant infection assays. G.S., X.K., X.H. and Y.W. performed DAB, ROS, salicylic acid, subcellular localization, RT–qPCR and GUS histochemical analyses. L.Y. and Z.Q. performed bioinformatics analysis. G.S., J.G., L.F., L.G., J.C.M., Y.B. and Q.L. performed lipidomics assays. Y.Z. and Y.W. performed chemical supplementation analyses of rbl1. G.S., Q.S., Q.G., Q. Zhou and T.-Y.C. performed yeast mutant complementation analyses. J.Z. and K.X. generated the CRISPR constructs. X.K., X.H., Y.L., W. Zhou, W. Zhang, Q. Zeng and Z.K. screened the edited lines. G.S., Y.W., R.H. and J.X. performed field trials and agronomic trait analyses. G.L. and G.S. drafted the manuscript and G.L., G.S., P.S., L.F., L.Z., L.G., K.X., J.C.M., Q.L., Y.B., Z.K. and P.C.R. revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
G.L., G.S. and X.H. are coinventors on a provisional patent application no. 202111041400.3 filed by Huazhong Agricultural University entitled ‘The OsRBL1 truncated protein and its application in balancing disease resistance and yield in rice’ that covers rbl1 and rbl1Δ12 lines. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature thanks Zuhua He and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 The plant glycerolipid metabolic pathway related to cytidinediphosphate-diacylglycerol synthase 1 (CDS1).
Rice RBL1 is homologous to yeast Cds1. CDP-DAG, cytidinediphosphate-diacylglycerol; DAG, diacylglycerol; PA, phosphatidic acid; PAH, phosphatidic acid phosphohydrolase; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PG, phosphatidylglycerol; PGPS, phosphatidylglycerol phosphate synthase; PI, phosphatidylinositol; PI4K, phosphatidylinositol 4-kinases; PI4P, phosphatidylinositol 4-phosphate; PI4P5K, phosphatidylinositol 4-phosphate 5-kinase; PI(4,5)P2, phosphatidylinositol-4,5-bisphosphate; PIS, phosphatidylinositol synthase; PS, phosphatidylserine; PSS, phosphatidylserine synthase.
Extended Data Fig. 2 Expression of plant defense-related genes, yield, and genetic complementation of the rbl1 line.
a, Panicles and seeds of the rbl1 and WT lines. Bars, 1 cm. Grain yield of the rbl1 and WT lines. Data are displayed as box and whisker plots with individual data points. The box plot elements are: center line, median; box limits, 25th and 75th percentiles. b, In situ detection of reactive oxygen species (ROS) in the rbl1 mutant and wild-type (WT, KitaakeX) leaves using 3,3′-diaminobenzidine (DAB) staining. Bar, 1 cm. c, Total salicylic acid (SA) levels are increased in the rbl1 mutant. Total SA was isolated from leaves of 2-week-old seedlings. d, qRT-PCR assays of genes that serve as markers of the plant immune response. Total RNA was extracted from leaves of 4-week-old plants. The Actin gene was used as the internal control. e, A 29 nucleotide deletion cosegregates with a lesion mimic phenotype in the M3 population derived from line rbl1. PCR results of InDel markers targeting the 29 nucleotide deletion: one short band, homozygous; one large band, wild-type alleles; two bands, heterozygous. “+” indicates lesions on the leaf of the M3 plant and “−” no lesion. A χ2 test of the phenotypic ratio revealed that the actual value 26: 92 of lesioned plants to normal plants is statistically similar to the expected value 1: 3 (χ2 = 0.213, P-value = 0.644 > 0.05). Similar results were obtained from three independent experiments. f, Complementation assays. Genotyping of the T0 lines using Cleaved Amplified Polymorphic Sequences (CAPS) markers. Leaves were photographed at 21 days post sowing (dps). For results of agarose gel electrophoresis, one band indicates the rbl1 mutant and three bands indicate the complemented line. Bar, 1 cm. Similar results were obtained from three independent experiments. g, qRT-PCR assays of RBL1 in the WT, rbl1, and complementation lines. Complementation line 10 was used as CoR1 in Fig. 1. The Actin gene was used as the internal control. Data are mean ± s.e.m, n = number of biologically independent samples in the graphs. Asterisks indicate significant differences compared to the WT using the two-tailed Student’s t-test (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001). For exact P values, see.
Extended Data Fig. 3 Expression assays of RBL1.
a, qRT-PCR assays of RBL1 in different tissues of the wild-type (WT, Kitaake) plants at the flowering stage. b, qRT-PCR assays of RBL1 in response to spray inoculation of M. oryzae strain ZB25 on Kitaake seedlings. c, Tissue-specific expression of RBL1 visualized by staining for the β-glucuronidase (GUS) reporter activity under control of the RBL1 promoter. For numbers: 1, seedling; 2 and 3, root; 4, stem; 5, shoot apical mristem; 6, leaf; 7, stamen; 8, pistil; 9, premilk stage; 10, panicle; 11, maturity stage. Bars in 1, 4, and 7, 5 mm; bars in 2, 3, 5, 6, and 8 to 11, 1 mm. Data are mean ± s.e.m, n = number of biologically independent samples in the graphs. Significant differences indicated by different letters were calculated using the Duncan’s new multiple range test.
Extended Data Fig. 4 Arabidopsis thaliana cds mutants show enhanced resistance to Phytophthora capsica.
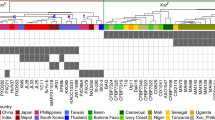
a, Phylogenetic analysis of RBL1 homologs from plants and other organisms. The phylogenetic tree was constructed using MEGA10. Accession numbers for different RBL1 homologs: Arabidopsis thaliana (NP_176433.2), Beta vulgaris subsp. vulgaris (XP_010694835.1), Brachypodium distachyon (XP_003564318.1), Brassica rapa (RID78417.1), Chenopodium quinoa (XP_021731901.1), Chlamydomonas reinhardtii (PNW85433.1), Glycine max (XP_003556374.1), Gossypium barbadense (KAB2026876.1), Homo sapiens (NP_001254.2), Hordeum vulgare (KAE8818711.1), Mus musculus (NP_775546.2), Oryza sativa (NC_029256.1), Saccharomyces cerevisiae (AJQ02739.1), Schizosaccharomyces pombe (NP_596416.1), Sorghum bicolor (KAG0539475.1), Triticum aestivum (KAF7023922.1), and Zea mays (NP_001132909.1). b, Protein domain analysis of RBL1 homologs from various organisms. Conserved protein domains in RBL1 homologs were predicted using SMART and visualized using iTOL. CTP_transf_1, phosphatide cytidylyltransferase. c, Amino acid alignment of the 19 residues that are truncated in the rbl1 line. The consensus is shown at the bottom. d, The cds mutant and wild-type (WT, Col-0, A. thaliana) plants at 28 days post sowing (dps). Bar, 1 cm. e, qRT-PCR assays. Total RNA was extracted from leaves of 4-week-old plants. f, Leaf length of the WT and cds mutant lines at 28 dps. g–i, Infected leaves (g), lesion area (h), and relative quantification of pathogen biomass (i) of WT and cds lines 36 h after inoculation (hpi) with Phytophthora capsica strain LT263. Bar, 1 cm. Data are mean ± s.e.m, n = number of biologically independent samples in the graphs. The box plot elements are: center line, median; box limits, 25th and 75th percentiles. Significant differences indicated by different letters were calculated using the Duncan’s new multiple range test.
Extended Data Fig. 5 Exogenous supplementation of PI delays lesion formation in rbl1.
a, Lesion formation was suppressed in the rbl1 but not cul3a or spl-D mutants. Plants were grown on 1/2 MS media supplemented with PI. WT, the wild-type Kitaake. Photographs were taken from 12-day-old rbl1, 2-week-old clu3a, and 2-week-old spl-D plants with corresponding control lines. Bar, 1 cm. b, Lesion formation in the rbl1 mutant was not affected by application of exogenous PG. Plants were grown on the 1/2 MS media supplemented with PG. Photographs were taken from 12-day-old plants. Bar, 1 cm. c, Lesion formation in the rbl1 mutant was enhanced with exogenous application of PI(4,5)P2. Plants were grown on the 1/2 MS media supplemented with PI(4,5)P2. Photographs were taken from 10-day-old plants. Bar, 1 cm. d, Exogenous PA enhances lesion formation in rbl1 and leaf tip necrosis in WT. Bar, 1 cm. e, Lesion formation in rbl1 was suppressed by exogenous diphenyleneiodonium chloride (DPI). Plants were grown on 1/2 MS media supplemented with DPI. Photographs were taken of 10-day-old plants. Bar, 1 cm. Data are mean ± s.e.m, n = number of independent replicates. Asterisks indicate significant differences compared to the mock using the two-tailed Student’s t-test (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001). For exact P values, see.
Extended Data Fig. 6 Design of multiplexed gRNAs for genome editing of RBL1 and field trials.
a, Gene structure of RBL1 and the site targeted by each numbered guide RNA (gRNA). b, Map and cloning sites of the CRISPR/Cas9 vector pRGEB32 used in genome editing (left); vectors with different gRNAs (right). c, Sequences of gRNAs designed using CRISPR-P 2.0. Purple letters indicate protospacer-adjacent motif sites (PAMs). d, A schematic diagram of co-transformation of different constructs and genotyping of T0 lines. Edited sites in each T0 plant were identified using Sanger sequencing and agarose gel electrophoresis. WT, the wild-type Kitaake. e, Design of the normal field plots. f, Design of the field plots in the rice blast nursery. WT, the wild-type Kitaake; rbl1Δ12, the edited line; LTH, the very susceptible rice variety Lijiangxintuanheigu, which was used as the spreader line for rice blast. Each plot contains 100 plants 0.2 m apart. g, Identification of transgene-free T1 plants of rbl1Δ12. Primers specific to the Cas9, hph and Actin genes, respectively, were used in genotyping. The hph gene encoding a hygromycin B phosphotransferase confers hygromycin resistance in the rice transgenic lines. The amplicon of the Actin gene was used as the DNA quality control. WT, Kitaake. Similar results were obtained from three independent experiments. h. Agronomic traits of the rbl1Δ12 and Kitaake lines. Data for each agronomic trait were collected from 50 plants for each line that was grown in the normal paddy field. Data are mean ± s.e.m, n = number of biologically independent samples in the graphs. In the box and whisker plots, dots indicate individual data points, and the error bars represent maximum and minimum values. Center line, median; box limits, 25th and 75th percentiles. Asterisks indicate significant differences using the two-tailed Student’s t-test (****P < 0.0001). For exact P values, see.
Extended Data Fig. 7 Characterization of the rbl1Δ12 line and the allele.
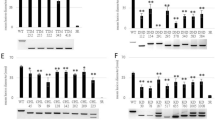
a, Sanger sequencing of the edited site indicated by the arrow in the rbl1Δ12 line with the wild-type (WT, Kitaake) as the reference. The PAM site was shown in blue, and the dashed line represents the 12 nucleotide deletion. b, Amino acid sequence alignment of RBL1 homologs around the four residues (underlined) that are truncated in the rbl1Δ12 line. Highlighted are conserved residues in the RBL1 homologs. c, ROS generation in the WT, rbl1Δ12, Nipponbare (Nip), LTH, and R498 rice plants challenged with chitin at the booting stage. RLU, relative light unit. d, qRT-PCR assays of RBL1 expression in different tissues of the WT, rbl1Δ12, and rbl1 plants at the flowering stage. e, qRT-PCR assays of heterologous expression of RBL1 in yeast. Yeast 18s rRNA was used as the internal control. f, Immunoblotting analysis of RBL1-, rbl1-, and RBL1Δ12-6×His fusion proteins in yeast. Ponceau S staining indicates the protein loading. WT, yeast strain BY4741; other strains are transformants of the yeast cds1 mutant carrying pYES2-RBL1, pYES2-rbl1, and pYES2- RBL1Δ12, respectively. Similar results were obtained from three independent experiments. g, Stability of the RBL1- and RBL1Δ12-6×His fusion proteins analyzed using differential scanning calorimetry (DSC). h, Subcellular localization of the RBL1- and RBL1Δ12-GFP fusion proteins transiently expressed in Nicotiana benthamiana leaf epidermal cells, analyzed together with the endoplasmic reticulum marker HDEL-mCherry. Bars, 25 μm. i, RBL1-GFP rescues the growth defect of yeast cds1. WT, yeast strain BY4741; R1G, the yeast cds1 mutant carrying pYES2-RBL1-GFP. Strains were cultured on YPGal or YPD plates at 30 °C for 3 days before sampling. Immunoblotting analysis of the RBL1-GFP fusion protein in the yeast strain R1G that forms a homodimer. CBB staining indicates the protein loading. Similar results were obtained from three independent experiments. j, 9-week-old WT, rbl1Δ12, and complemented T1 plants. The white asterisk indicates the spontaneous lesion in the insets. Bar, 10 cm. Shown on the right are qRT-PCR assays of RBL1 in the WT, rbl1Δ12, and CoR1:: rbl1Δ12 lines. The Actin gene was used as the internal control. k, Punch inoculation of the WT, rbl1Δ12, and CoR1:: rbl1Δ12 lines with M. oryzae. The lesion area was measured at 14 dpi. Bar, 1 cm. l, Leaves of 3-week-old WT, rbl1, rbl1Δ12, and F1 plants of rbl1 crossed with rbl1Δ12. Bar, 1 cm. m, The WT, rbl1Δ12, and F2 plants derived from the WT line crossed with rbl1Δ12 at 60 dps. Spontaneous lesions-indicated by white asterisks-formed on the top leaves of homozygous rbl1Δ12 lines. Bars, 10 cm. n, Punch inoculation assays of the WT, rbl1Δ12, and F2 plants with M. oryzae. The lesion area was measured at 14 dpi. Bar, 1 cm. o, qRT-PCR assays of RBL1 in the WT, rbl1Δ12 and F2 plants at the tillering stage. p, WT and rbl1Δ12 transgenic plants expressing the PI(4,5)P2 biosensor at 32 hpi with M. oryzae stain ZB25 expressing the cytoplasmic effector Pwl2 tagged with mCherry. BIC, biotrophic interfacial complex; EIHM, extra-invasive hyphal membrane; IH, invasive hyphae. Bar, 10 μm. q, BIC formation in plants shown in (p). r, Membrane lipid composition analysis of the WT, rbl1Δ12, and rbl1 lines. DW, dry weight. s, PIP and PIP2 content in the WT, rbl1Δ12, and rbl1 lines. Data are mean ± s.e.m, n = number of biologically independent samples in the graphs. The box plot elements are: center line, median; box limits, 25th and 75th percentiles. Asterisks in (e) and (q) indicate significant differences using the two-tailed Student’s t-test (*P < 0.05, **P < 0.01). For exact P values, see Source Data. Significant differences indicated by different letters in (d),(j),(k), (n),(o),(r), and (s) were calculated using the Duncan’s new multiple range test.
Extended Data Fig. 8 No mutations were observed in predicted off-target sites in rbl1Δ12.
The top seven predicted off-target sites were analyzed using Sanger sequencing with the Kitaake (WT) genome as the reference. Purple letters indicate protospacer-adjacent motif sites (PAMs). The right panel shows the sequencing results of the potential off-target sites.
Extended Data Fig. 9 Genome editing of RBL1 enhances disease resistance in two other rice cultivars.
a, Lesion mimic phenotypes and enhanced resistance to M. oryzae in RBL1-edited Nipponbare (Nip) lines. Infected leaves and lesion area of punch-inoculated RBL1-edited lines with M. oryzae at 14 dpi. Bar, 1 cm. b, 12-week-old RBL1-edited Nipponbare lines. Bar, 10 cm. c, qRT-PCR assays of RBL1 and plant defense-related genes OsPR8 and OsPR10 in the RBL1-edited Nipponbare lines. Total RNA was extracted from 4-week-old leaves. The Actin gene was used as the internal control. d–f, Similar assays as shown in (a–c) were performed on rice cultivar Zhonghua11 (ZH11). g, Sanger sequencing of the edited sites, indicated by arrows, in nr-7 and zr-7 lines, with the wild-type (WT, Kitaake) as the reference. The mutated nucleotides are shown in gray (deletion) and blue (insertion). h, Amino acid sequence alignment of the region mutated in lines nr-7 and zr-7. The four amino acids truncated in RBL1Δ12 are indicated by arrows. Shaded are conserved residues in the RBL1 homologs. Two amino acids are truncated in nr-7, with the first one overlapping the truncated residues in RBL1Δ12. An 84-bp frameshift mutation caused by a 1-bp deletion followed by a 1-bp insertion alters the sequence of 28 amino acids (gray) in zr-7, with the first two overlapping the truncated residues in RBL1Δ12. Data are mean ± s.e.m, n = number of biologically independent samples in the graphs. And asterisks indicate significant differences compared to the WT using the unpaired Student’s t-test (**P < 0.01, ***P < 0.001, ****P < 0.0001). For exact P values, see Source Data. Significant differences indicated by different letters in (c) and (f) were calculated using the Duncan’s new multiple range test.
Supplementary information
Supplementary Fig. 1
Uncropped gel and immunoblotting images for main and Extended Data Figures 2, 6 and 7.
Supplementary Table 1
RT–qPCR assays of OsPIS1 in OsPIS1::rbl1 lines at the tillering stage. RNA was isolated from leaf tissues. The Actin gene was used as the internal control. Data are mean ± s.e.m., n = the number of biologically independent samples in the graphs.
Supplementary Table 2
Primers used in this study.
Source data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sha, G., Sun, P., Kong, X. et al. Genome editing of a rice CDP-DAG synthase confers multipathogen resistance. Nature 618, 1017–1023 (2023). https://doi.org/10.1038/s41586-023-06205-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-06205-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.