Abstract
Stromal cells in adult bone marrow that express leptin receptor (LEPR) are a critical source of growth factors, including stem cell factor (SCF), for the maintenance of haematopoietic stem cells and early restricted progenitors1,2,3,4,5,6. LEPR+ cells are heterogeneous, including skeletal stem cells and osteogenic and adipogenic progenitors7,8,9,10,11,12, although few markers have been available to distinguish these subsets or to compare their functions. Here we show that expression of an osteogenic growth factor, osteolectin13,14, distinguishes peri-arteriolar LEPR+ cells poised to undergo osteogenesis from peri-sinusoidal LEPR+ cells poised to undergo adipogenesis (but retaining osteogenic potential). Peri-arteriolar LEPR+osteolectin+ cells are rapidly dividing, short-lived osteogenic progenitors that increase in number after fracture and are depleted during ageing. Deletion of Scf from adult osteolectin+ cells did not affect the maintenance of haematopoietic stem cells or most restricted progenitors but depleted common lymphoid progenitors, impairing lymphopoiesis, bacterial clearance, and survival after acute bacterial infection. Peri-arteriolar osteolectin+ cell maintenance required mechanical stimulation. Voluntary running increased, whereas hindlimb unloading decreased, the frequencies of peri-arteriolar osteolectin+ cells and common lymphoid progenitors. Deletion of the mechanosensitive ion channel PIEZO1 from osteolectin+ cells depleted osteolectin+ cells and common lymphoid progenitors. These results show that a peri-arteriolar niche for osteogenesis and lymphopoiesis in bone marrow is maintained by mechanical stimulation and depleted during ageing.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Source data files are provided with this paper. RNA sequencing data have been submitted to the NCBI Sequence Read Archive (SRA), accession number BioProject PRJNA626582. Source data are provided with this paper.
References
Ding, L., Saunders, T. L., Enikolopov, G. & Morrison, S. J. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 481, 457–462 (2012).
Ding, L. & Morrison, S. J. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature 495, 231–235 (2013).
Oguro, H., Ding, L. & Morrison, S. J. SLAM family markers resolve functionally distinct subpopulations of hematopoietic stem cells and multipotent progenitors. Cell Stem Cell 13, 102–116 (2013).
Cordeiro Gomes, A. et al. Hematopoietic stem cell niches produce lineage-instructive signals to control multipotent progenitor differentiation. Immunity 45, 1219–1231 (2016).
Himburg, H. A. et al. Distinct bone marrow sources of pleiotrophin control hematopoietic stem cell maintenance and regeneration. Cell Stem Cell 23, 370–381 (2018).
Comazzetto, S. et al. Restricted hematopoietic progenitors and erythropoiesis require SCF from leptin receptor+ niche cells in the bone marrow. Cell Stem Cell 24, 477–486 (2019).
Zhou, B. O., Yue, R., Murphy, M. M., Peyer, J. G. & Morrison, S. J. Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell 15, 154–168 (2014).
Zhou, B. O. et al. Bone marrow adipocytes promote the regeneration of stem cells and haematopoiesis by secreting SCF. Nat. Cell Biol. 19, 891–903 (2017).
Tikhonova, A. N. et al. The bone marrow microenvironment at single-cell resolution. Nature 569, 222–228 (2019).
Baryawno, N. et al. A cellular taxonomy of the bone marrow stroma in homeostasis and leukemia. Cell 177, 1915–1932 (2019).
Baccin, C. et al. Combined single-cell and spatial transcriptomics reveal the molecular, cellular and spatial bone marrow niche organization. Nat. Cell Biol. 22, 38–48 (2020).
Matsushita, Y. et al. A Wnt-mediated transformation of the bone marrow stromal cell identity orchestrates skeletal regeneration. Nat. Commun. 11, 332 (2020).
Yue, R., Shen, B. & Morrison, S. J. Clec11a/osteolectin is an osteogenic growth factor that promotes the maintenance of the adult skeleton. eLife 5, e18782 (2016).
Shen, B. et al. Integrin alpha11 is an Osteolectin receptor and is required for the maintenance of adult skeletal bone mass. eLife 8, e42274 (2019).
Crane, G. M., Jeffery, E. & Morrison, S. J. Adult haematopoietic stem cell niches. Nat. Rev. Immunol. 17, 573–590 (2017).
Greenbaum, A. et al. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature 495, 227–230 (2013).
Saçma, M. et al. Haematopoietic stem cells in perisinusoidal niches are protected from ageing. Nat. Cell Biol. 21, 1309–1320 (2019).
Young, K. et al. Progressive alterations in multipotent hematopoietic progenitors underlie lymphoid cell loss in aging. J. Exp. Med. 213, 2259–2267 (2016).
Kusumbe, A. P. et al. Age-dependent modulation of vascular niches for haematopoietic stem cells. Nature 532, 380–384 (2016).
Kiel, M. J. et al. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 121, 1109–1121 (2005).
Acar, M. et al. Deep imaging of bone marrow shows non-dividing stem cells are mainly perisinusoidal. Nature 526, 126–130 (2015).
Christodoulou, C. et al. Live-animal imaging of native haematopoietic stem and progenitor cells. Nature 578, 278–283 (2020).
Visnjic, D. et al. Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood 103, 3258–3264 (2004).
Zhu, J. et al. Osteoblasts support B-lymphocyte commitment and differentiation from hematopoietic stem cells. Blood 109, 3706–3712 (2007).
Kusumbe, A. P., Ramasamy, S. K. & Adams, R. H. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature 507, 323–328 (2014).
Kalajzic, Z. et al. Directing the expression of a green fluorescent protein transgene in differentiated osteoblasts: comparison between rat type I collagen and rat osteocalcin promoters. Bone 31, 654–660 (2002).
Dobnig, H. & Turner, R. T. Evidence that intermittent treatment with parathyroid hormone increases bone formation in adult rats by activation of bone lining cells. Endocrinology 136, 3632–3638 (1995).
Park, D. et al. Endogenous bone marrow MSCs are dynamic, fate-restricted participants in bone maintenance and regeneration. Cell Stem Cell 10, 259–272 (2012).
Bianco, P., Kuznetsov, S. A., Riminucci, M. & Gehron Robey, P. Postnatal skeletal stem cells. Methods Enzymol. 419, 117–148 (2006).
Morrison, S. J., Wandycz, A. M., Akashi, K., Globerson, A. & Weissman, I. L. The aging of hematopoietic stem cells. Nat. Med. 2, 1011–1016 (1996).
Ladel, C. H., Flesch, I. E., Arnoldi, J. & Kaufmann, S. H. Studies with MHC-deficient knock-out mice reveal impact of both MHC I- and MHC II-dependent T cell responses on Listeria monocytogenes infection. J. Immunol. 153, 3116–3122 (1994).
Kayraklioglu, N., Horuluoglu, B., Elango, M. & Klinman, D. M. Critical role of B cells in Toll-like receptor 7-mediated protection against Listeria monocytogenes infection. Infect. Immun. 87, e00742-19 (2019).
Robling, A. G. et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J. Biol. Chem. 283, 5866–5875 (2008).
Li, X. et al. Stimulation of Piezo1 by mechanical signals promotes bone anabolism. eLife 8, e49631 (2019).
Sun, W. et al. The mechanosensitive Piezo1 channel is required for bone formation. eLife 8, e47454 (2019).
Iwaniec, U. T. & Turner, R. T. Influence of body weight on bone mass, architecture and turnover. J. Endocrinol. 230, R115–R130 (2016).
Coste, B. et al. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 330, 55–60 (2010).
Wang, L. et al. Mechanical sensing protein PIEZO1 regulates bone homeostasis via osteoblast-osteoclast crosstalk. Nat. Commun. 11, 282 (2020).
Coste, B. et al. Piezo proteins are pore-forming subunits of mechanically activated channels. Nature 483, 176–181 (2012).
Syeda, R. et al. Chemical activation of the mechanotransduction channel Piezo1. eLife 4, e07369 (2015).
Boron, W. F. & Boulpaep, E. L. Medical Physiology 2nd edn. Ch. 19 (Saunders, 2009).
Grüneboom, A. et al. A network of trans-cortical capillaries as mainstay for blood circulation in long bones. Nat. Metab. 1, 236–250 (2019).
Sivaraj, K. K. & Adams, R. H. Blood vessel formation and function in bone. Development 143, 2706–2715 (2016).
DeFalco, J. et al. Virus-assisted mapping of neural inputs to a feeding center in the hypothalamus. Science 291, 2608–2613 (2001).
Madisen, L. et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 13, 133–140 (2010).
Daigle, T. L. et al. A suite of transgenic driver and reporter mouse lines with enhanced brain-cell-type targeting and functionality. Cell. 174, 465–480 (2018).
Cahalan, S. M. et al. Piezo1 links mechanical forces to red blood cell volume. eLife 4, e07370 (2015).
Muzumdar, M. D., Tasic, B., Miyamichi, K., Li, L. & Luo, L. A global double-fluorescent Cre reporter mouse. Genesis 45, 593–605 (2007).
Morrison, S. J. et al. Culture in reduced levels of oxygen promotes clonogenic sympathoadrenal differentiation by isolated neural crest stem cells. J. Neurosci. 20, 7370–7376 (2000).
Gao, D. et al. Activation of cyclic GMP–AMP synthase by self-DNA causes autoimmune diseases. Proc. Natl Acad. Sci. USA 112, E5699–E5705 (2015).
Acknowledgements
S.J.M. is a Howard Hughes Medical Institute (HHMI) Investigator, the Mary McDermott Cook Chair in Pediatric Genetics, the Kathryn and Gene Bishop Distinguished Chair in Pediatric Research, the director of the Hamon Laboratory for Stem Cells and Cancer, and a Cancer Prevention and Research Institute of Texas Scholar. This work was supported partly by the National Institutes of Health (DK118745 to S.J.M.). B.S. was supported by a Ruth L. Kirschstein National Research Service Award Postdoctoral Fellowship from the National Heart, Lung, and Blood Institute (F32 HL139016-01). A.T. was supported by the Leopoldina Fellowship Program (LPDS 2016-16) of the German National Academy of Sciences and the Fritz Thyssen Foundation. L.A.J. was supported by ARC Discovery grants (DP200101970, DE190100609). R.S. was supported by the UT Southwestern Medical Center Endowed Scholar Program, Welch Foundation Award (I-1965-20180324) and an American Heart Association scientist development grant (17SDG33410184). We thank H. Zeng and H. Taniguchi for providing the Ai47 mice; N. Loof, T. Shih and the Moody Foundation Flow Cytometry Facility; and the BioHPC high-performance computing cloud at University of Texas Southwestern Medical Center for providing computational resources.
Author information
Authors and Affiliations
Contributions
B.S. and S.J.M. conceived the project, designed and interpreted experiments. B.S. performed most of the experiments, with technical assistance and discussions with A.T., J.M.U. and G.M.C. J.Z. and M.M.M. developed immunofluorescence staining protocols. E.D.N and R.S. performed the electrophysiological recordings measuring PIEZO1 channel activity. S.H. assisted in Listeria infection experiments. L.D. and N.K. helped to perform flow cytometry. Y.Y. and Z.H. assisted in establishing the hindlimb unloading model and helped with image acquisition. S.G., Y.J. and X.L. assisted B.S. to perform RNA sequencing and analysis. V.R. assisted in ossicle transplantation in NSG mice. E.C.M. and C.E. performed genotyping on mice. L.A.J. and Y.C.Z. provided advice on the mechanical properties of arterioles. Z.Z. performed bioinformatic and statistical analyses. B.S. and S.J.M. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature thanks Iannis Aifantis, Sara Wickstrom and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 OlnmT reporter mice showed that osteolectin is expressed by osteoblasts, osteocytes, and chondrocytes in addition to peri-arteriolar LEPR+ stromal cells.
a, The mouse Oln gene was modified to insert an mTomato-WPRE-pA cassette between the 5′ untranslated region and exon 3 to generate the targeting vector. These sites were selected to avoid disrupting conserved intronic sequences. Open boxes indicate untranslated regions and black boxes indicate translated regions of Oln. b, The targeted founder mouse (F0) was identified by Southern blotting using 5′ and WPRE probes (black bars). c, PCR genotyping of genomic DNA confirmed germline transmission of the OlnmT allele. Mice were backcrossed at least three times onto a C57BL/Ka background before analysis. d, Oln transcript levels by qPCR with reverse transcription (RT–qPCR) in Oln-mTomato+ and Oln-mTomato− bone marrow cells (3 mice analysed in 3 independent experiments). e, Flow cytometry gates to distinguish Oln-mTomato+LEPR+ from Oln-mTomato−LEPR+ stromal cells in enzymatically dissociated bone marrow. f, Oln transcript levels by RT–qPCR in Oln-mTomato+LEPR+ and Oln-mTomato−LEPR+ bone marrow cells (4 mice analysed in 4 independent experiments). g, We did not detect Oln-Tomato in VE-cadherin-expressing bone marrow endothelial cells (4 mice per genotype analysed in 4 independent experiments). h, i, Femur epiphysis (h) and diaphysis (i) from OlnmT/+;Col1a1*2.3-eGFP mice (images are representative of 3 independent experiments from 8- to 10-week-old mice). In h, the arrowhead points to Oln-tdTomato+Col1a1*2.3-eGFP+ osteoblasts in trabecular bone and the arrow points to Oln-tdTomato+Col1a1*2.3-eGFP− hypertrophic chondrocytes in the growth plate (scale bar, 100 μm). i, Oln-tdTomato+Col1a1*2.3-eGFP+ osteoblasts at the endosteum (arrow), Oln-tdTomato+ osteocytes (arrowhead), and Oln-tdTomato+ periosteal cells (asterisk) (scale bars, 40 μm). All data represent mean ± s.d.
Extended Data Fig. 2 Bone marrow Oln-mTomato+ cells localized mainly around arterioles in the diaphysis.
a, A low-magnification view of the femur diaphysis showed Oln-mTomato+ stromal cells associated with arterioles enriched in the centre of the marrow. In this image, arteriolar and sinusoidal blood vessels were distinguished based on size, morphology and continuity of the basal lamina, visualized using anti-laminin antibody staining as previously described21 (images are representative of 3 independent experiments; scale bar, 200 μm). b, OlnmT/+ femur bone marrow showing that, in contrast to the diaphysis (Fig. 1h), most SCA-1+ arterioles were not surrounded by Oln-mTomato+ stromal cells in the metaphysis. The Oln-mTomato+ cells in this panel were osteoblasts and osteocytes associated with trabecular bone (scale bar, 500 μm). c, d, Gene set enrichment analysis showing significant enrichment of genes associated with osteogenesis in CD45−Ter119−CD31−Scf-GFP+Oln-mTomato+ stromal cells and adipogenesis in CD45−Ter119−CD31−Scf-GFP+Oln-mTomato− stromal cells (FDR, false discovery rate; NES, normalized enrichment score; 4 mice analysed in 4 independent experiments). e, f, The mouse Oln gene was modified by inserting an iCreER-WPRE-pA cassette between the 5′ untranslated region and exon 3 to generate the targeting vector (e). These sites were selected to avoid disrupting conserved intronic sequences. Open boxes indicate untranslated regions and black boxes indicate translated regions of Oln. f, The targeted founder mouse (F0) was identified by Southern blotting using 5′ and WPRE probes (black bars). g, PCR genotyping of genomic DNA confirmed germline transmission of the OlniCreER allele. Mice were backcrossed at least three times onto a C57BL/Ka background before analysis. h, Deep imaging of OlniCreER/+;Rosa26loxp-tdTomato/+ femur bone marrow 3 days after tamoxifen administration at 2 months of age, showing that Oln-tdTomato+ cells were exclusively peri-arteriolar in the diaphysis (images are representative of 3 independent experiments; scale bar, 200 μm). i, j, Deep imaging of the femur epiphysis (i) and diaphysis (j) one month after tamoxifen administration at 2 months of age (images are representative of 3 independent experiments). i, Oln-tdTomato+ hypertrophic chondrocytes in the growth plate (arrow) and Oln-tdTomato+Col1a1*2.3-eGFP+ osteoblasts in trabecular bone (arrowhead; scale bar, 60 μm). j, Oln-tdTomato+Col1a1*2.3-eGFP+ osteoblasts at the endosteum (arrow), an Oln-tdTomato+ osteocyte (arrowhead), and Oln-tdTomato+ periosteal cells (asterisk; scale bars, 30 μm). k, Col1a1-CreER;Rosa26loxp-tdTomato/+;Col1a1*2.3-eGFP mice were treated with tamoxifen at 2 months of age and the percentage of Col1a1*2.3-eGFP+ osteoblasts that were tdTomato+ was assessed 3 days to 1 month later (3 mice per time point analysed in 3 independent experiments). All data represent mean ± s.d.
Extended Data Fig. 3 Osteolectin+ cells create a niche for CLPs but not other haematopoietic stem or progenitor cells.
a, Two-month-old OlniCreER/+;Rosa26loxp-tdTomato/+ mice were sublethally irradiated 3 days after tamoxifen administration. Two weeks later, none of the adipocytes in the bone marrow were tdTomato+ (image representative of 3 independent experiments; scale bar, 60 μm). b, Representative H&E stained sections from ossicles formed by Oln-mTomato+ (left) or Oln-mTomato– (right) stromal cells sorted from OlnmT/+ mice showing bone (arrowheads), haematopoietic cells (arrow), and adipocytes (asterisk; images are representative of 5 independent experiments; scale bar, 100 μm). c–e, OlniCreER/+;Scffl/fl and Scffl/fl littermate controls were treated with tamoxifen at two months of age. One month later, blood from OlniCreER/+; Scffl/fl mice showed normal white blood cell (c), red blood cell (d) and platelet (e) counts (6 mice per genotype analysed in three independent experiments). f–k, OlniCreER/+;Scffl/fl mice and littermate controls exhibited no significant differences in the frequencies of B220+ B cells (f), CD3+ T cells (g), Gr-1+Mac-1+ myeloid cells (h), CD41+ megakaryocyte lineage cells (i), CD71+/Ter119+ erythroid lineage cells (j) and HSCs or MPPs in the spleen (k; 6 mice per genotype analysed in 3 independent experiments). l, Bone marrow cells from OlniCreER/+;Scffl/fl mice and littermate controls gave similar levels of donor cell reconstitution upon competitive transplantation into irradiated mice (5 donor mice per genotype analysed in 3 independent experiments). The differences between OlniCreER/+;Scffl/fl and Scffl/fl mice were not statistically significant in c–l. m, OlnmT/+;ScfGFP/+ femur bone marrow showing Oln-mTomato+ osteoblasts at the endosteum were negative for Scf-GFP, while peri-arteriolar Oln-mTomato+ stromal cells were positive for Scf-GFP (representative of 3 independent experiments; scale bar, 80 μm). n, OlnmT/+;Col1a1*2.3-eGFP femur bone marrow showing Col1a1*2.3-eGFP+ osteoblasts at the endosteum were Oln-mTomato+ (representative of 3 independent experiments; scale bar, 100 μm). All data represent mean ± s.d. Statistical significance was assessed using matched-samples two-way ANOVA followed by Sidak’s (c–e, k, l) or Tukey’s (f–j) multiple-comparisons tests.
Extended Data Fig. 4 Flow cytometry gating strategy for the isolation of haematopoietic stem and progenitor cell populations.
a–d, Representative flow cytometry gates used to identify the haematopoietic stem and progenitor cell populations in the bone marrow (a, b), and T lineage progenitors in the thymus (c, d). The markers used to identify each of the cell populations characterized in this study are listed in Supplementary Table 1. e, Representative flow cytometry gates showing that more than 50% of IL7Rα+lineage− cells were Lin−SCA1lowKITlowIL7Rα+Flt3+ CLPs and most of the non-CLP IL7Rα+lineage− (Flt3−) cells were CD19+, probably other early B lineage progenitors (4 mice analysed in 3 independent experiments). All data represent mean ± s.d.
Extended Data Fig. 5 Oln-mTomato+ osteoblasts and osteocytes in the metaphysis do not express Scf-GFP and Oln-mTomato+ cells in the diaphysis expand during fracture healing.
The differences among treatments in cell frequencies were also evident in absolute numbers. (a–c) Epiphysis (a) and metaphysis (b, c) of OlnmT/+;ScfGFP/+ femur bone marrow showing that hypertrophic chondrocytes (arrowhead in a; scale bars, 30μm) as well as osteoblasts and osteocytes associated with trabecular bone (arrowhead in c) were negative for Scf-GFP (images are representative of 3 independent experiments; scale bars, 400 μm (b) and 100 μm (c)). Most of the Oln-Tomato staining in the metaphysis reflects Col1a1*2.3-GFP+ osteoblasts and osteocytes associated with trabecular bone, as shown in Fig. 1d and Extended Data Fig. 2b. The boxed area in b is magnified in c. d, Image of the metaphysis of ScfGFP/+ femur bone marrow showing limited non-specific staining by anti-tdTomato antibody (scale bar, 100 μm). e–g, OlniCreER/+;Scffl/fl and Scffl/fl littermate control mice were fed tamoxifen chow from 2–4 months of age. They exhibited no significant differences in the frequencies of HSCs, MPPs, GMPs, MEPs, or CMPs in the bone marrow (e), B220+ B cells (f), or CD3+ T cells (g) in the bone marrow or spleen. The differences between OlniCreER/+; Scffl/fl and Scffl/fl mice were not statistically significant in e–g (5 mice per genotype analysed in 3 independent experiments). h, i, Three days after tamoxifen, femurs were fractured in two-month-old OlnmT/iCreER;Scffl/fl mice and OlnmT/+;Scffl/fl littermate controls then the bone marrow was analysed two weeks later. i, HSC and MPP frequencies did not significantly change during fracture healing. h, By contrast, CLP frequencies significantly increased in OlnmT/+;Scffl/fl control but not in OlnmT/iCreER;Scffl/fl mice. The frequency of osteolectin+ cells significantly increased in both OlnmT/iCreER;Scffl/fl mice and OlnmT/+;Scffl/fl controls (i); 5 mice per genotype analysed in 3 independent experiments). j, Localization of IL7Rα+Lineage– lymphoid progenitors in the marrow of the fractured as compared to control (CON) femur (4 mice per treatment analysed in 4 independent experiments). All data represent mean ± s.d. Statistical significance was assessed using matched-samples two-way ANOVAs followed by Sidak’s (e–i) or Tukey’s multiple-comparisons tests (h), or Cochran–Mantel–Haenszel test (j).
Extended Data Fig. 6 CLPs and peri-arteriolar osteolectin+ cells are depleted during ageing but most other haematopoietic stem and progenitor cell populations are not.
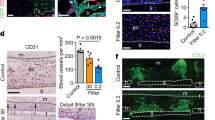
The differences among treatments in cell frequencies were also evident in absolute numbers. a–d, OlnmT/+ mice received daily subcutaneous injections with PBS or 40 μg/kg human PTH for 28 days. PTH treated mice exhibited significantly thicker cortical bone (a, b) and significant reductions in the frequencies of osteolectin+ cells (c) and CLPs (d) (8 mice per treatment analysed in 4 independent experiments). Micro CT images (b; scale bar, 800 μm) of cortical bone are representative of 8 independent experiments. e, f, The frequency of LEPR+ cells (e) and the percentage of LEPR+ cells that were Oln-mTomato+ (f) in OlnmT/+ bone marrow at 2 to 18 months of age (8 mice per time point analysed in 4 independent experiments). g, Femur bone marrow from an 18-month-old OlnmT/+ mouse showing an arteriole surrounded by Oln-mTomato+ cells (arrowhead) and an arteriole lacking Oln-mTomato+ cells (arrow) in the diaphysis (image is representative of 4 independent experiments; scale bar, 100 μm). h, The frequency of CLPs in OlnmT/+ mice at 2 to 18 months of age. i–m, During ageing, the frequencies of HSCs (i) and MPPs (j) in the bone marrow increased while the frequencies of GMPs (k), CMPs (l), and MEPs (m) did not significantly change (8 mice per time point analysed in 4 independent experiments). n, Localization of IL7Rα+Lineage− cells in the bone marrow of 2- and 18-month-old mice (7 mice per time point analysed in 3 independent experiments). o, Ageing significantly depleted osteolectin+ cells associated with arterioles in both endosteal and non-endosteal regions of diaphysis bone marrow (8 mice per time point analysed in 4 independent experiments). p, Number of IL7Rα+Lineage− cells per 100 μm of osteolectin+ or osteolectin− arteriole at 2 and 18 months of age (7 mice per time point analysed in 3 independent experiments). All data represent mean ± s.d. Statistical significance was assessed using paired t-tests (a, c, d, o, p), one-way ANOVA followed by Dunnett’s multiple-comparisons test (e), matched-samples two-way ANOVAs followed by Dunnett’s multiple-comparisons tests (f, h–m), Cochran–Mantel–Haenszel test (n) or Mann–Whitney tests followed by Holm–Sidak’s multiple-comparisons adjustments (o).
Extended Data Fig. 7 OlniCreER/+;Scffl/fl mice exhibited reduced lymphopoiesis and impaired bacterial clearance after Listeria infection.
The same differences that were evident among treatments in the frequencies of cell populations were also evident in absolute numbers. a–c, OlniCreER/+;Scffl/fl mice and littermate controls were treated with tamoxifen at 2 months of age and then 3 days later were administered Listeria orally. Relative to Scffl/fl littermate controls, OlniCreER/+;Scffl/fl mice had decreased B (a) and T (b) cell counts in the spleen 5 days after Listeria infection and increased bacterial CFUs in the spleen 10 days after infection (c; 11-16 mice per genotype analysed in 3 independent experiments). d−k, OlniCreER/+;Scffl/fl and Scffl/fl littermate controls were fed tamoxifen from 2 to 4-months of age then 3 days later were administered Listeria intraperitoneally. Relative to Scffl/fl littermate controls, OlniCreER/+;Scffl/fl mice exhibited reduced frequencies of pre-proB cells, but not proB or preB cells, in the bone marrow (d) as well as ETPs and DN1 cells in the thymus (e) at 5 and/or 10 days after infection. OlniCreER/+;Scffl/fl mice also exhibited reduced numbers of NK cells in the spleen (f) as well as B cells (g), T cells (h), and NK cells (i) in mesenteric lymph nodes (mLN) at 5 and 10 days after infection. j, OlniCreER/+;Scffl/fl mice exhibited increased bacterial CFUs in mLN at 5 and 10 days after infection. k, The percentage of CD3+ T cells that were IFNγ+ did not significantly differ between the spleens of OlniCreER/+;Scffl/fl mice and littermate controls (18–27 mice per genotype analysed in 3 independent experiments). l–n, Compared to Scffl/fl controls, OlniCreER/+;Scffl/fl mice had decreased spleen B (l) and T (m) cell counts, and increased spleen bacterial CFUs (n) at 5 and 10 days after infection with Listeria (21–27 mice per genotype analysed in 3 independent experiments). All data represent mean ± s.d. Statistical significance was assessed using matched-samples two-way ANOVA followed by Sidak’s multiple-comparisons tests (a, b, d–n) or Wilcoxon matched-pairs test (c).
Extended Data Fig. 8 Mechanical stimulation is required for the maintenance of peri-arteriolar, but not peri-sinusoidal, niches for lymphoid progenitors in the bone marrow.
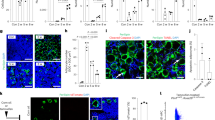
a, The effect of voluntary running for 4 weeks on femoral cortical bone mineral density (6 mice per treatment analysed in 3 independent experiments). b, Voluntary running for 4 weeks did not significantly affect the frequencies of HSCs, MPPs, GMPs, MEPs, CMPs, or CLPs in calvarium bone marrow (5 mice per treatment analysed in 3 independent experiments). c–h, Hindlimb unloading (c) for 2 weeks did not significantly affect forelimb cortical bone mineral density (d), cortical thickness (e), the percentage of LEPR+ cells that were Oln-mTomato+ (f), or the frequencies of HSCs, MPPs, GMPs, MEPs, CMPs or CLPs in humerus bone marrow (g). h, Hindlimb unloading for 2 weeks significantly reduced hindlimb (femur) cortical bone mineral density (5 mice per treatment analysed in 3 independent experiments). i, All point current amplitude histograms of the electrical recordings in Fig. 4h. The single channel conductance was 15 ± 1 pS from the Gaussian fits to the amplitude histograms. The y-axis shows the number of events. j, k, Single channel current recordings and corresponding all point current histograms of Piezo1 deficient Oln-mTomato+ cells isolated from OlnmT/iCreER;Piezo1fl/fl mice. The data were collected at -60 mm Hg applied pressure and at the holding potential of +60 mV, without (j) or with (k) 40 μM Yoda1 in the pipette. l, Piezo1 transcript levels by RT–qPCR in Oln-mTomato+ cells from OlnmT/iCreER;Piezo1fl/fl and OlnmT/+;Piezo1fl/fl control mice (6 mice per genotype analysed in 3 independent experiments). m, n, The effect of Piezo1 deletion in osteolectin+ cells on femoral cortical bone mineral density (m) and the percentage of osteolectin+ cells that incorporated a 48-h pulse of BrdU (n; 6 mice per genotype analysed in 3 independent experiments). o, Location of IL7Rα+Lineage− cells in the bone marrow of OlnmT/iCreER;Piezo1fl/fl and OlnmT/+;Piezo1fl/fl control mice (6 mice per genotype analysed in 3 independent experiments). p–s, We treated Col1a1-CreER;Piezo1fl/fl;OlnmT/+ mice and littermate controls with tamoxifen at 2 months of age and analysed them 1 month later. Piezo1 deletion in osteoblasts significantly reduced femur bone mineral density (p) and cortical thickness (q) but not the frequencies of osteolectin+ cells (r) or HSCs, MPPs, GMPs, MEPs or CLPs in the bone marrow (s; 5–6 mice per genotype analysed in 3 independent experiments). t–w, We analysed the femurs of Leprcre/+;Piezo1fl/fl;OlnmT/+ mice and littermate controls at 4 months of age and found that Piezo1 deletion in LEPR+ cells did not significantly reduce bone mineral density (t) but did reduce cortical thickness (u). Piezo1 deletion in LEPR+ cells also significantly reduced the frequencies of osteolectin+ cells (v) and CLPs (w) without affecting the frequencies of HSCs, MPPs, GMPs, MEPs or CMPs (w) in the bone marrow (5 mice per genotype analysed in 3 independent experiments). x–z, Compared to Scffl/fl littermate controls, OlniCreER/+;Piezo1fl/fl mice had decreased B (x) and T (y) cell counts in the spleen 5 days after oral Listeria infection and increased bacterial CFUs in the spleen 10 days after infection (z; 11-16 mice per genotype analysed in 3 independent experiments). All data represent mean ± s.d. Statistical significance was assessed using paired t-tests (a, l, m, n, p–r, t–v, z), matched-samples two-way ANOVAs followed by Sidak’s (b, d–f, h, s, w–y) or Holm–Sidak’s multiple-comparisons adjustment (g), or Cochran-Mantel–Haenszel test (o).
Supplementary information
Supplementary Figure 1
Uncropped scans with size marker indications. For Western blots, each protein was analyzed on a separate gel.
Supplementary Table 1
Summary of the markers used by flow cytometry to identify each of the cell populations characterized in this study.
Supplementary Table 2
FPKM values from RNA-seq analysis of mesenchymal, niche, osteogenic, and adipogenic genes in CD45−Ter119−CD31−Scf-GFP+Oln-mTomato+ and CD45−Ter119−CD31−Scf-GFP+Oln-mTomato− bone marrow stromal cells (cell samples were isolated from 4 mice on 4 different days). All data represent mean ± SD.
Source data
Rights and permissions
About this article
Cite this article
Shen, B., Tasdogan, A., Ubellacker, J.M. et al. A mechanosensitive peri-arteriolar niche for osteogenesis and lymphopoiesis. Nature 591, 438–444 (2021). https://doi.org/10.1038/s41586-021-03298-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-021-03298-5
This article is cited by
-
PIEZO1 loss-of-function compound heterozygous mutations in the rare congenital human disorder Prune Belly Syndrome
Nature Communications (2024)
-
Clonal hematopoiesis and its impact on the aging osteo-hematopoietic niche
Leukemia (2024)
-
Resilient anatomy and local plasticity of naive and stress haematopoiesis
Nature (2024)
-
Cellular niches for hematopoietic stem cells in bone marrow under normal and malignant conditions
Inflammation and Regeneration (2023)
-
Leptin receptor+ cells promote bone marrow innervation and regeneration by synthesizing nerve growth factor
Nature Cell Biology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.