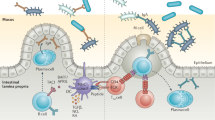
Abstract
Alterations in enteric microbiota are associated with several highly prevalent immune-mediated and metabolic diseases1,2,3, and experiments involving faecal transplants have indicated that such alterations have a causal role in at least some such conditions4,5,6. The postnatal period is particularly critical for the development of microbiota composition, host–microbe interactions and immune homeostasis7,8,9. However, the underlying molecular mechanisms of this neonatal priming period have not been defined. Here we report the identification of a host-mediated regulatory circuit of bacterial colonization that acts solely during the early neonatal period but influences life-long microbiota composition. We demonstrate age-dependent expression of the flagellin receptor Toll-like receptor 5 (TLR5) in the gut epithelium of neonate mice. Using competitive colonization experiments, we demonstrate that epithelial TLR5-mediated REG3γ production is critical for the counter-selection of colonizing flagellated bacteria. Comparative microbiota transfer experiments in neonate and adult wild-type and Tlr5-deficient germ-free mice reveal that neonatal TLR5 expression strongly influences the composition of the microbiota throughout life. Thus, the beneficial microbiota in the adult host is shaped during early infancy. This might explain why environmental factors that disturb the establishment of the microbiota during early life can affect immune homeostasis and health in adulthood.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Change history
29 August 2018
In Fig. 1d of this Letter, the third group along should have been labelled ‘WT’ rather than ‘Tlr5’. This has been corrected online.
References
Turnbaugh, P. J. et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444, 1027–1031 (2006).
Qin, J. et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 490, 55–60 (2012).
Frank, D. N. et al. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl Acad. Sci. USA 104, 13780–13785 (2007).
Henao-Mejia, J. et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 482, 179–185 (2012).
Schaubeck, M. et al. Dysbiotic gut microbiota causes transmissible Crohn’s disease-like ileitis independent of failure in antimicrobial defence. Gut 65, 225–237 (2015).
Vijay-Kumar, M. et al. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science 328, 228–231 (2010).
Olszak, T. et al. Protective mucosal immunity mediated by epithelial CD1d and IL-10. Nature 509, 497–502 (2014).
Cahenzli, J., Köller, Y., Wyss, M., Geuking, M. B. & McCoy, K. D. Intestinal microbial diversity during early-life colonization shapes long-term IgE levels. Cell Host Microbe 14, 559–570 (2013).
Wesemann, D. R. et al. Microbial colonization influences early B-lineage development in the gut lamina propria. Nature 501, 112–115 (2013).
Bäckhed, F. et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe 17, 852 (2015).
Yatsunenko, T. et al. Human gut microbiome viewed across age and geography. Nature 486, 222–227 (2012).
Dominguez-Bello, M. G. et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl Acad. Sci. USA 107, 11971–11975 (2010).
Faith, J. J. et al. The long-term stability of the human gut microbiota. Science 341, 1237439 (2013).
Torow, N. & Hornef, M. W. The neonatal window of opportunity: Setting the stage for life-long host-microbial interaction and immune homeostasis. J. Immunol. 198, 557–563 (2017).
Pott, J. et al. Age-dependent TLR3 expression of the intestinal epithelium contributes to rotavirus susceptibility. PLoS Pathog. 8, e1002670 (2012).
Zhang, K. et al. Age-dependent enterocyte invasion and microcolony formation by Salmonella. PLoS Pathog. 10, e1004385 (2014b).
Ubeda, C. et al. Familial transmission rather than defective innate immunity shapes the distinct intestinal microbiota of TLR-deficient mice. J. Exp. Med. 209, 1445–1456 (2012).
Chassaing, B., Koren, O., Carvalho, F. A., Ley, R. E. & Gewirtz, A. T. AIEC pathobiont instigates chronic colitis in susceptible hosts by altering microbiota composition. Gut 63, 1069–1080 (2014).
Uematsu, S. & Akira, S. Immune responses of TLR5+ lamina propria dendritic cells in enterobacterial infection. J. Gastroenterol. 44, 803–811 (2009).
Oh, J. Z. et al. TLR5-mediated sensing of gut microbiota is necessary for antibody responses to seasonal influenza vaccination. Immunity 41, 478–492 (2014).
Kinnebrew, M. A. et al. Bacterial flagellin stimulates Toll-like receptor 5-dependent defense against vancomycin-resistant Enterococcus infection. J. Infect. Dis. 201, 534–543 (2010).
Kinnebrew, M. A. et al. Interleukin 23 production by intestinal CD103+CD11b+ dendritic cells in response to bacterial flagellin enhances mucosal innate immune defense. Immunity 36, 276–287 (2012).
Carvalho, F. A. et al. Transient inability to manage proteobacteria promotes chronic gut inflammation in TLR5-deficient mice. Cell Host Microbe 12, 139–152 (2012).
Vijay-Kumar, M. et al. Deletion of TLR5 results in spontaneous colitis in mice. J. Clin. Invest. 117, 3909–3921 (2007).
Singh, V. et al. Microbiota-dependent hepatic lipogenesis mediated by stearoyl CoA desaturase 1 (SCD1) promotes metabolic syndrome in TLR5-deficient mice. Cell Metab. 22, 983–996 (2015).
Lantier, L. et al. Poly(I:C)-induced protection of neonatal mice against intestinal Cryptosporidium parvum infection requires an additional TLR5 signal provided by the gut flora. J. Infect. Dis. 209, 457–467 (2014).
Andersen-Nissen, E. et al. Evasion of Toll-like receptor 5 by flagellated bacteria. Proc. Natl Acad. Sci. USA 102, 9247–9252 (2005).
Uematsu, S. et al. Detection of pathogenic intestinal bacteria by Toll-like receptor 5 on intestinal CD11c+ lamina propria cells. Nat. Immunol. 7, 868–874 (2006).
Chassaing, B., Ley, R. E. & Gewirtz, A. T. Intestinal epithelial cell toll-like receptor 5 regulates the intestinal microbiota to prevent low-grade inflammation and metabolic syndrome in mice. Gastroenterology 147, 1363–1377.e17 (2014).
Zhang, B. et al. Viral infection. Prevention and cure of rotavirus infection via TLR5/NLRC4-mediated production of IL-22 and IL-18. Science 346, 861–865 (2014).
Lodes, M. J. et al. Bacterial flagellin is a dominant antigen in Crohn disease. J. Clin. Invest. 113, 1296–1306 (2004).
Gewirtz, A. T. et al. Dominant-negative TLR5 polymorphism reduces adaptive immune response to flagellin and negatively associates with Crohn’s disease. Am. J. Physiol. Gastrointest. Liver Physiol. 290, G1157–G1163 (2006).
Bank, S. et al. Polymorphisms in the Toll-like receptor and the IL-23/IL-17 pathways were associated with susceptibility to inflammatory bowel disease in a Danish cohort. PLoS ONE 10, e0145302 (2015).
Datsenko, K. A. & Wanner, B. L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl Acad. Sci. USA 97, 6640–6645 (2000).
Barthel, M. et al. Pretreatment of mice with streptomycin provides a Salmonella enterica serovar Typhimurium colitis model that allows analysis of both pathogen and host. Infect. Immun. 71, 2839–2858 (2003).
Lotz, M. et al. Postnatal acquisition of endotoxin tolerance in intestinal epithelial cells. J. Exp. Med. 203, 973–984 (2006).
Sommer, F. et al. Altered mucus glycosylation in core 1 O-glycan-deficient mice affects microbiota composition and intestinal architecture. PLoS ONE 9, e85254 (2014).
Segata, N. et al. Metagenomic biomarker discovery and explanation. Genome Biol. 12, R60 (2011).
Simon, R. & Samuel, C. E. Activation of NF-κB-dependent gene expression by Salmonella flagellins FliC and FljB. Biochem. Biophys. Res. Commun. 355, 280–285 (2007).
Acknowledgements
We thank D. Gütle, T. Albers, M. Nietschke, A. Smoczek, V. Tremaroli and M. Krämer for technical support and M. Rühlemann for discussions. The work was supported by the priority programs SPP1656 (Ho-2236/9-1 and BL953/5-1) and SPP1580 (Ho 2236/11-1, He 1964/18-2), SFB944 (P4 to M.H.) and SFB1182 (C2 to F.S. and P.R.) and the single grant Ho 2236/14-1 from the German Research Foundation (DFG), as well as the Lower Saxony-Israel Fond and the Niedersachsen-Research Network on Neuroinfectiology (N-RENNT) of the Ministry of Science and Culture of Lower Saxony, Germany, to M.F. and M.W.H. M.F. and K.v.V. were supported by the German Federal Ministry of Education and Research (BMBF) within the consortium InfectControl 2020 (Project NeoBiom, grant ID 03ZZ0829C). M.F. was supported by the Freie Universität Berlin within the Excellence Initiative of the DFG and P.R. by the ExC306 Inflammation at Interfaces. B.C. is supported by a Career Development Award from the Crohn’s and Colitis Foundation and an Innovator Award from the Kenneth Rainin Foundation. F.B. is Torsten Söderberg Professor in Medicine and recipient of ERC consolidator Grant 2013 (European Research Council, Consolidator grant 615362-METABASE).
Reviewer information
Nature thanks J. Kagan and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
M.F. and M.W.H. performed and analysed colonization and infection experiments. M.F., M.B., B.C., A.B. and A.T.G. planned and performed microbiota transfer experiments. M.F., M.B., B.C. and A.D. performed conventional and germ-free mouse breeding and sample collection. F.S., F.B., P.R., and M.F. analysed the enteric microbiota samples. K.v.V., M.F. and A.D. analysed faecal flagellin content. M.H. generated bacterial mutants. R.K. performed histopathological analysis. M.W.H., A.T.G. and F.B. supervised the study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Role of TLR5 and flagellin expression in the pathogenesis of neonatal Salmonella infection.
a, b, Intestinal colonization and organ dissemination evaluated by serial dilution and plating two (a) or three (b) days after infection of 1-day-old wild-type (WT, filled circles, n = 5) or Tlr5-deficient mice (Tlr5, open circles, n = 4 or n = 5, respectively) with approximately 102 CFU S. Typhimurium. Results from one litter at each time point. c, Intestinal colonization and organ dissemination evaluated by serial dilution and plating in small intestine (n = 6 and 11), colon (n = 7 and 11), liver (n = 10 and 12), spleen (n = 10 and 12) and lung (n = 10 and 12) 4 days after infection of 1-day-old WT (filled symbols) mice with approximately 102 WT S. Typhimurium (circles, two litters) or ΔfljB/fliC mutant S. Typhimurium (triangles, three litters). d, Intestinal colonization and organ dissemination evaluated by serial dilution and plating in small intestine (n = 6 and 4), colon (n = 9 and 4), liver (n = 9 and 4), spleen (n = 9 and 4) and lung (n = 9 and 4) 4 days after infection of 1-day-old Tlr5-deficient (open symbols) mice with approximately 102 WT S. Typhimurium (circles, 1 litter) or ΔfljB/fliC mutant S. Typhimurium (triangles, 1 litter). Data in a–d represent the median (two-sided Mann–Whitney test). e, Survival of 1-day-old WT mice infected orally with wild-type (wt, dashed line, n = 20, three litters) or isogenic flagellin-deficient (ΔfljB/fliC, solid line, n = 22, three litters) S. Typhimurium. f, Cxcl2 mRNA expression analysed by quantitative RT–PCR in intestinal epithelial cells (IECs) isolated 4 days after infection of 1-day-old wild-type mice (WT, filled symbols, n = 13 and 11 from each three litters) or Tlr5-deficient mice (Tlr5, open symbols, n = 6 and 4 from each two litters) with wild-type S. Typhimurium (wt, circles) or an isogenic flagellin mutant (ΔfljB/fliC, triangles). Values were normalized to the expression of the housekeeping gene Hprt. Data represent the median (*P < 0.05; **P < 0.01, two-sided Mann–Whitney test).
Extended Data Fig. 2 Kinetics of counter-selection against flagellated Salmonella in Tlr5-proficient mice.
Intestinal colonization (CI) at 3, 6, 12, and 24 h after oral infection of 1-day-old mice with a 1:1 ratio of wild-type and ΔfljB/fliC S. Typhimurium. Data represent the median; n = 7, 7, 7 (derived from 2 litters) and 26 (6 litters), respectively; **P < 0.01, Kruskal–Wallis test followed by Dunn’s post-test). Note that results from competition experiments with WT and flagellin-deficient (ΔfljB/fliC) S. Typhimurium in WT mice after 24 h presented in Fig. 1d, f, h and Extended Data Fig. 2 are identical and were pooled from six independent experiments conducted in two different laboratory animal facilities.
Extended Data Fig. 3 Influence of TLR5 on microbiota composition and the metabolic phenotype in individually housed adult mice.
a, Bacterial DNA was extracted from the faeces of individually housed 6–8-week-old homozygous Tlr5+/+ (WT, red, n = 8) and homozygous Tlr5−/− (Tlr5, blue, n = 8) mice. The microbiota composition was analysed by 16S rDNA sequencing and the overall difference is illustrated in a principal coordinate analysis (PCoA). b, Body weight was evaluated in 7-week-old male WT (red) and Tlr5-deficient (blue) mice. Data represent the median. Statistical analysis was performed using two-sided Mann–Whitney U-test (n = 6 and 12, respectively, **P < 0.01). c, Daily food intake was evaluated in 25-week-old female WT (red) and Tlr5-deficient (blue) mice. Data represent the median. Statistical analysis was performed using two-sided Mann–Whitney U-test (n = 3 and 4, respectively). d, e, Haematoxylin and eosin staining of liver (d) and pancreas tissue sections (e) from 18-week-old WT and Tlr5-deficient mice. Images were randomly selected from n = 2 (WT) and n = 3 (Tlr5-deficient) mice examined. d, In contrast to WT mice, liver tissue from Tlr5-deficient mice showed decreased eosinophilic staining intensity of hepatocytes located close to the central veins (black asterisk) that is caused by an accumulation of microvacuoles in their cytoplasm consistent with increased lipid storage (black arrows). Scale bars: i and iii, 100 µm; ii and iv, 50 µm. e, Pancreas tissue from WT mice contained more and larger islets of Langerhans (black asterisks) than tissue derived from Tlr5-deficient mice. In addition, exocrine epithelial cells from WT mice contained larger amounts of eosinophilic zymogen granules (black arrows) than the more basophilic cells of Tlr5-deficient mice. Scale bars: i and iii; 100 µm; ii and iv, 20 µm.
Extended Data Fig. 4 Neonatal but not adult epithelial Reg3g mRNA expression is influenced by TLR5 expression.
IECs from non-infected wild-type (WT, red) and Tlr5-deficient (Tlr5, blue) mice were collected at 1 (n = 9 from three litters and n = 7 from three litters), 4 (n = 7 from two litters and n = 10 from two litters), 10 (n = 9 from one litter and n = 11 from two litters) and 21 days (n = 9 from one litter and n = 6 from one litter) after birth and Reg3g mRNA expression was analysed by qRT–PCR. Values were normalized to the expression of the housekeeping gene Hprt. Data represent the median (*P < 0.05; **P < 0.01; ***P < 0.001; two-sided Mann–Whitney U-test).
Extended Data Fig. 5 REG3γ expression influences the faecal bioactive flagellin load.
Faecal pellets from age-matched WT (n = 10; 6 female, 4 male) and Reg3g-deficient (n = 13; 8 female, 5 male) 5–8-week-old mice housed in 3 and 4 separate isolator cages, respectively, were collected. The amount of bioactive, pro-inflammatory flagellin was determined using the HEK-Blue-mTLR5 cell line as described in the Methods section. Data represent the median from two independent measurements; *P < 0.05, two-sided Mann–Whitney U-test.
Supplementary information
Supplementary Tables
This file contains Supplementary Table 1 (Salmonella enterica serovar Typhimurium strains used in this study) and Supplementary Table 2 (Oligonucleotides used in this study).
Rights and permissions
About this article
Cite this article
Fulde, M., Sommer, F., Chassaing, B. et al. Neonatal selection by Toll-like receptor 5 influences long-term gut microbiota composition. Nature 560, 489–493 (2018). https://doi.org/10.1038/s41586-018-0395-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-018-0395-5
This article is cited by
-
Composition, function, and timing: exploring the early-life gut microbiota in piglets for probiotic interventions
Journal of Animal Science and Biotechnology (2023)
-
Gut–liver axis: barriers and functional circuits
Nature Reviews Gastroenterology & Hepatology (2023)
-
Microbiota-dependent presence of murine enteric glial cells requires myeloid differentiation primary response protein 88 signaling
Journal of Biosciences (2023)
-
The potential application of probiotics for the prevention and treatment of COVID-19
Egyptian Journal of Medical Human Genetics (2022)
-
Should we modulate the neonatal microbiome and what should be the goal?
Microbiome (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.