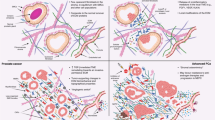
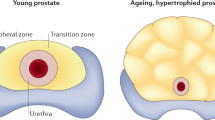
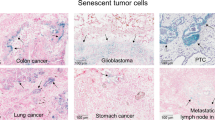
Abstract
The stromal component of the tumour microenvironment in primary and metastatic prostate cancer can influence and promote disease progression. Within the prostatic stroma, fibroblasts are one of the most prevalent cell types associated with precancerous and cancerous lesions; they have a vital role in the structural composition, organization and integrity of the extracellular matrix. Fibroblasts within the tumour microenvironment can undergo cellular senescence, which is a stable arrest of cell growth and a phenomenon that is emerging as a recognized hallmark of cancer. Supporting the idea that cellular senescence has a pro-tumorigenic role, a subset of senescent cells exhibits a senescence-associated secretory phenotype (SASP), which, along with increased inflammation, can promote prostate cancer cell growth and survival. These cellular characteristics make targeting senescent cells and/or modulating SASP attractive as a potential preventive or therapeutic option for prostate cancer.
Key points
-
Cellular senescence is a stable arrest of cell growth and is emerging as a recognized hallmark of cancer.
-
Senescent fibroblasts in the prostatic stroma can exhibit a senescence-associated secretory phenotype (SASP) in the tumour microenvironment, which can contribute to increased local inflammation, thereby promoting prostate cancer development, progression and resistance to therapy.
-
To eliminate the ability of senescent cells to produce factors that promote cancer cell growth and survival, specifically targeting the senescent cells with senotherapeutics would be an attractive option in prostate cancer treatment.
-
The preventive or therapeutic potential of using senotherapeutics could most benefit those disproportionately affected by prostate cancer (such as Black men and men with considerable comorbidities) or men with prostate cancer who are at high risk of disease progression.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Sung, H. et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021).
Siegel, R. L., Miller, K. D., Fuchs, H. E. & Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 72, 7–33 (2022).
Siegel, R. L., Miller, K. D., Wagle, N. S. & Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 73, 17–48 (2023).
National Cancer Institute. Prostate: recent trends in U.S. age-adjusted mortality rates, 2000–2019(a). SEER https://seer.cancer.gov/statistics-network/explorer/application.html?site=66&data_type=2&graph_type=2&compareBy=age_range&chk_age_range_1=1&chk_age_range_160=160&chk_age_range_166=166&hdn_sex=2&race=1&advopt_precision=1&advopt_show_ci=on&hdn_view=0&advopt_show_apc=on&advopt_display=2 (2020).
Siegel, D. A., O’Neil, M. E., Richards, T. B., Dowling, N. F. & Weir, H. K. Prostate cancer incidence and survival, by stage and race/ethnicity — United States, 2001–2017. Morb. Mortal. Wkly Rep. 69, 1473–1480 (2020).
Mori, J. O. et al. Novel forms of prostate cancer chemoresistance to successful androgen deprivation therapy demand new approaches: rationale for targeting BET proteins. Prostate 82, 1005–1015 (2022).
Mori, J. O. et al. Molecular and pathological subtypes related to prostate cancer disparities and disease outcomes in African American and European American patients. Front. Oncol. 12, 928357 (2022).
Cancer tomorrow. World Health Organization https://gco.iarc.fr/tomorrow (2021).
Coppé, J. P., Desprez, P. Y., Krtolica, A. & Campisi, J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu. Rev. Pathol. 5, 99–118 (2010).
Fiard, G. et al. Cellular senescence as a possible link between prostate diseases of the ageing male. Nat. Rev. Urol. 18, 597–610 (2021). Overview linking benign prostate hyperplasia and prostate cancer to senescent cells found in the ageing prostate.
Hayflick, L. & Moorhead, P. S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 25, 585–621 (1961).
Harley, C. B., Futcher, A. B. & Greider, C. W. Telomeres shorten during ageing of human fibroblasts. Nature 345, 458–460 (1990).
Artandi, S. E. & DePinho, R. A. A critical role for telomeres in suppressing and facilitating carcinogenesis. Curr. Opin. Genet. Dev. 10, 39–46 (2000).
Huang, W., Hickson, L. T. J., Eirin, A., Kirkland, J. L. & Lerman, L. O. Cellular senescence: the good, the bad and the unknown. Nat. Rev. Nephrol. 18, 611–627 (2022).
Van Deursen, J. M. The role of senescent cells in ageing. Nature 509, 439–446 (2014).
Jun, J. I. L. & Lau, L. F. The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nat. Cell Biol. 12, 676–685 (2010).
Birch, J. & Gil, J. Senescence and the SASP: many therapeutic avenues. Genes Dev. 34, 1565–1576 (2020).
Myrianthopoulos, V. et al. Senescence and senotherapeutics: a new field in cancer therapy. Pharmacol. Ther. 193, 31–49 (2019).
Coppé, J. P. et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 6, 2853–2868 (2008). These findings outline features of genotoxic stress-induced senescence and suggest cell-nonautonomous mechanisms for the development of cancer by altering the tissue microenvironment.
Krtolica, A., Parrinello, S., Lockett, S., Desprez, P. Y. & Campisi, J. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc. Natl Acad. Sci. USA 98, 12072–12077 (2001).
Bavik, C. et al. The gene expression program of prostate fibroblast senescence modulates neoplastic epithelial cell proliferation through paracrine mechanisms. Cancer Res. 66, 794–802 (2006).
Begley, L., Monteleon, C., Shah, R. B., Macdonald, J. W. & Macoska, J. A. CXCL12 overexpression and secretion by aging fibroblasts enhance human prostate epithelial proliferation in vitro. Aging Cell 4, 291–298 (2005). In vitro studies with stromal fibroblastic cells isolated from patients demonstrate that the ageing prostatic stroma enhances prostate epithelial cell proliferation in a paracrine manner through the CXCL12–CXCR4 signalling axis.
Dimri, G. P. et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl Acad. Sci. USA 92, 9363–9367 (1995).
Waaijer, M. E. C. et al. The number of p16INK4a positive cells in human skin reflects biological age. Aging Cell 11, 722–725 (2012).
Tuttle, C. S. L. et al. Cellular senescence and chronological age in various human tissues: a systematic review and meta-analysis. Aging Cell 19, e13083 (2020).
Hernandez-Segura, A., Nehme, J. & Demaria, M. Hallmarks of cellular senescence. Trends Cell Biol. 28, 436–453 (2018). This work describes senescence-phenotype molecular regulators and how the regulators could be used to identify senescent cells in vitro and in vivo.
Basisty, N. et al. A proteomic atlas of senescence-associated secretomes for aging biomarker development. PLoS Biol. 18, e3000599 (2020). This describes a proteomics database of senescence inducers and molecules secreted by senescent cells of different cell types.
Sikora, E., Bielak-Zmijewska, A. & Mosieniak, G. What is and what is not cell senescence. Adv. Biochem. 64, 110–118 (2018).
da Silva, P. F. L. et al. The bystander effect contributes to the accumulation of senescent cells in vivo. Aging Cell 18, e12848 (2019). Mouse dermal fibroblasts and myofibres in the vicinity of senescent cell xenotransplants displayed an increased senescence phenotype compared with non-senescent cell xenotransplants.
Hanahan, D. Hallmarks of cancer: new dimensions. Cancer Discov. 12, 31–46 (2022).
Lau, L. & David, G. Pro- and anti-tumorigenic functions of the senescence-associated secretory phenotype. Expert Opin. Ther. Targets 23, 1041–1051 (2019).
He, S. & Sharpless, N. E. Senescence in health and disease. Cell 169, 1000–1011 (2017).
Matjusaitis, M., Chin, G., Sarnoski, E. A. & Stolzing, A. Biomarkers to identify and isolate senescent cells. Ageing Res. Rev. 29, 1–12 (2016).
Kudlova, N., De Sanctis, J. B. & Hajduch, M. Cellular senescence: molecular targets, biomarkers, and senolytic drugs. Int. J. Mol. Sci. 23, 4168 (2022).
Vermeulen, K., Van Bockstaele, D. R. & Berneman, Z. N. The cell cycle: a review of regulation, deregulation and therapeutic targets in cancer. Cell Prolif. 36, 131–149 (2003).
Beauséjour, C. M. et al. Reversal of human cellular senescence: roles of the p53 and p16 pathways. EMBO J. 22, 4212–4222 (2003).
Kotake, Y., Yaxue, Z. & Xiong, Y. DDB1-CUL4 and MLL1 mediate oncogene-induced p16INK4a activation. Cancer Res. 69, 1809–1814 (2009).
Stein, G. H., Drullinger, L. F., Soulard, A. & Dulić, V. Differential roles for cyclin-dependent kinase inhibitors p21 and p16 in the mechanisms of senescence and differentiation in human fibroblasts. Mol. Cell. Biol. 19, 2109–2117 (1999).
Herbig, U., Wei, W., Dutriaux, A., Jobling, W. A. & Sedivy, J. M. Real-time imaging of transcriptional activation in live cells reveals rapid up-regulation of the cyclin-dependent kinase inhibitor gene CDKN1A in replicative cellular senescence. Aging Cell 2, 295–304 (2003).
Wei, W., Hemmer, R. M. & Sedivy, J. M. Role of p14 ARF in replicative and induced senescence of human fibroblasts. Mol. Cell Biol. 21, 6748–6757 (2001).
Engeland, K. Cell cycle regulation: p53–p21–RB signaling. Cell Death Differ. 29, 946–960 (2022).
Serrano, M., Hannon, G. J. & Beach, D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature 366, 704–707 (1993).
Cayo, A. et al. mTOR activity and autophagy in senescent cells, a complex partnership. Int. J. Mol. Sci. 22, 8149 (2021).
Young, A. R. J. et al. Autophagy mediates the mitotic senescence transition. Genes Dev. 23, 798–803 (2009).
Merchut-Maya, J. M. & Maya-Mendoza, A. The contribution of lysosomes to DNA replication. Cells 10, 1068 (2021).
D’Adda Di Fagagna, F. et al. A DNA damage checkpoint response in telomere-initiated senescence. Nature 426, 194–198 (2003).
Gorgoulis, V. et al. Cellular senescence: defining a path forward. Cell 179, 813–827 (2019).
Kiyono, T. et al. Both Rb/p16INK4a inactivation and telomerase activity are required to immortalize human epithelial cells. Nature 396, 84–88 (1998).
Ramirez, R. D. et al. Putative telomere-independent mechanisms of replicative aging reflect inadequate growth conditions. Genes Dev. 15, 398–403 (2001).
Levesque, C. & Nelson, P. S. Cellular constituents of the prostate stroma: key contributors to prostate cancer progression and therapy resistance. Cold Spring Harb. Perspect. Med. 8, a030510 (2018). Overview highlighting how the reactive stroma changes in composition as a cause or consequence of intrinsic and extrinsic factors coevolves with prostate cancer progression.
Brennen, W. N., Denmeade, S. R. & Isaacs, J. T. Mesenchymal stem cells as a vector for the inflammatory prostate microenvironment. Endocr. Relat. Cancer 20, R269–R290 (2013).
Brennen, W. N., Kisteman, L. N. & Isaacs, J. T. Rapid selection of mesenchymal stem and progenitor cells in primary prostate stromal cultures. Prostate 76, 552–564 (2016).
Brennen, W. N. et al. Mesenchymal stem cell infiltration during neoplastic transformation of the human prostate. Oncotarget 8, 46710–46727 (2017).
Brennen, W. N. & Isaacs, J. T. Mesenchymal stem cells and the embryonic reawakening theory of BPH. Nat. Rev. Urol. 15, 703–715 (2018).
Sanches, B. D. A. et al. Stromal cell interplay in prostate development, physiology, and pathological conditions. Prostate 81, 926–937 (2021).
Henry, G. H. et al. A cellular anatomy of the normal adult human prostate and prostatic urethra. Cell Rep. 25, 3530–3542.e5 (2018).
Tuxhorn, J. A. et al. Reactive stroma in human prostate cancer: induction of myofibroblast phenotype and extracellular matrix remodeling. Clin. Cancer Res. 8, 2912–2923 (2002).
Rimal, R. et al. Cancer-associated fibroblasts: origin, function, imaging, and therapeutic targeting. Adv. Drug Deliv. Rev. 189, 114504 (2022).
Bedeschi, M., Marino, N., Cavassi, E., Piccinini, F. & Tesei, A. Cancer-associated fibroblast: role in prostate cancer progression to metastatic disease and therapeutic resistance. Cells 12, 802 (2023).
Owen, J. S., Clayton, A. & Pearson, H. B. Cancer-associated fibroblast heterogeneity, activation and function: implications for prostate cancer. Biomolecules 13, 67 (2023).
Barron, D. A. & Rowley, D. R. The reactive stroma microenvironment and prostate cancer progression. Endocr. Relat. Cancer 19, R187 (2012).
Fibroblast. National Human Genomic Research Institute https://www.genome.gov/genetics-glossary/Fibroblast (2023).
Wei, K., Nguyen, H. N. & Brenner, M. B. Fibroblast pathology in inflammatory diseases. J. Clin. Invest. 131, e149538 (2021).
Boesch, M. et al. Fibroblasts in cancer: defining target structures for therapeutic intervention. Biochim. Biophys. Acta Rev. Cancer 1872, 111–121 (2019).
Lynch, M. D. & Watt, F. M. Fibroblast heterogeneity: implications for human disease. J. Clin. Investig. 128, 26–35 (2018).
Plikus, M. V. et al. Fibroblasts: origins, definitions, and functions in health and disease. Cell 184, 3852–3872 (2021).
Koliaraki, V., Prados, A., Armaka, M. & Kollias, G. The mesenchymal context in inflammation, immunity and cancer. Nat. Immunol. 21, 974–982 (2020).
Davidson, S. et al. Fibroblasts as immune regulators in infection, inflammation and cancer. Nat. Rev. Immunol. 21, 704–717 (2021).
Gelse, K., Pöschl, E. & Aigner, T. Collagens — structure, function, and biosynthesis. Adv. Drug Deliv. Rev. 55, 1531–1546 (2003).
Shiao, S. L., Chu, G. C. Y. & Chung, L. W. K. Regulation of prostate cancer progression by the tumor microenvironment. Cancer Lett. 380, 340–348 (2016).
Kang, J. et al. Tumor microenvironment mechanisms and bone metastatic disease progression of prostate cancer. Cancer Lett. 530, 156–169 (2022). The primary tumour microenvironment and the premetastatic niche promote prostate cancer metastasis and therapy resistance.
Brennen, W. N., Isaacs, J. T. & Denmeade, S. R. Rationale behind targeting fibroblast activation protein-expressing carcinoma-associated fibroblasts as a novel chemotherapeutic strategy. Mol. Cancer Ther. 11, 257–266 (2012).
Sasaki, T., Franco, O. E. & Hayward, S. W. Interaction of prostate carcinoma-associated fibroblasts with human epithelial cell lines in vivo. Differentiation 96, 40–48 (2017).
Brennen, W. N. et al. Overcoming stromal barriers to immuno-oncological responses via fibroblast activation protein-targeted therapy. Immunotherapy 13, 155–175 (2021).
Puré, E. & Blomberg, R. Pro-tumorigenic roles of fibroblast activation protein in cancer: back to the basics. Oncogene 37, 4343–4357 (2018).
Erdogan, B. et al. Cancer-associated fibroblasts promote directional cancer cell migration by aligning fibronectin. J. Cell Biol. 216, 3799–3816 (2017).
ChallaSivaKanaka, S., Vickman, R. E., Kakarla, M., Hayward, S. W. & Franco, O. E. Fibroblast heterogeneity in prostate carcinogenesis. Cancer Lett. 525, 76–83 (2022).
Liao, Z., Tan, Z. W., Zhu, P. & Tan, N. S. Cancer-associated fibroblasts in tumor microenvironment — accomplices in tumor malignancy. Cell Immunol. 343, 103729 (2019).
Hu, B. & Phan, S. H. Myofibroblasts. Curr. Opin. Rheumatol. 25, 71–77 (2013).
Tai, Y. et al. Myofibroblasts: function, formation, and scope of molecular therapies for skin fibrosis. Biomolecules 11, 1095 (2021).
Chowdhury, R. et al. Cancer exosomes trigger mesenchymal stem cell differentiation into pro-angiogenic and pro-invasive myofibroblasts. Oncotarget 6, 715–731 (2015).
Hintz, H. M., Cowan, A. E., Shapovalova, M. & LeBeau, A. M. Development of a cross-reactive monoclonal antibody for detecting the tumor stroma. Bioconjug. Chem. 30, 1466–1476 (2019).
Hayward, S. W. Immunotherapeutic response in tumors is affected by microenvironmental ROS. Cancer Res. 80, 1799–1800 (2020).
Krueger, T. E., Thorek, D. L. J., Meeker, A. K., Isaacs, J. T. & Brennen, W. N. Tumor-infiltrating mesenchymal stem cells: drivers of the immunosuppressive tumor microenvironment in prostate cancer? Prostate 79, 320–330 (2019).
Andersen, M. K. et al. Integrative metabolic and transcriptomic profiling of prostate cancer tissue containing reactive stroma. Sci. Rep. 8, 14269 (2018).
Tuxhorn, J. A., McAlhany, S. J., Dang, T. D., Ayala, G. E. & Rowley, D. R. Stromal cells promote angiogenesis and growth of human prostate tumors in a differential reactive stroma (DRS) xenograft model. Cancer Res. 62, 3298–3307 (2002).
Tuxhorn, J. A., Ayala, G. E. & Rowley, D. R. Reactive stroma in prostate cancer progression. J. Urol. 166, 2472–2483 (2001).
Brennen, W. N. et al. Assessing angiogenic responses induced by primary human prostate stromal cells in a three-dimensional fibrin matrix assay. Oncotarget 7, 71298–71308 (2016).
Kfoury, Y. & Scadden, D. T. Mesenchymal cell contributions to the stem cell niche. Cell Stem Cell 16, 239–253 (2015).
Ridge, S. M., Sullivan, F. J. & Glynn, S. A. Mesenchymal stem cells: key players in cancer progression. Mol. Cancer 16, 31 (2017).
Lee, C. H., Moioli, E. K. & Mao, J. J. in Ann. Int. Conf. IEEE Engineering in Medicine and Biology Proc. Vol. 1, 775–778 (NIH Public Access, 2006).
Rahimi Tesiye, M., Abrishami Kia, Z. & Rajabi-Maham, H. Mesenchymal stem cells and prostate cancer: a concise review of therapeutic potentials and biological aspects. Stem Cell Res. 63, 102864 (2022).
Fu, X. et al. Mesenchymal stem cell migration and tissue repair. Cells 8, 784 (2019).
Pittenger, M. F. et al. Mesenchymal stem cell perspective: cell biology to clinical progress. npj Regen. Med. 4, 22 (2019).
Lama, V. N. et al. Evidence for tissue-resident mesenchymal stem cells in human adult lung from studies of transplanted allografts. J. Clin. Invest. 117, 989–996 (2007).
Hass, R., Kasper, C., Böhm, S. & Jacobs, R. Different populations and sources of human mesenchymal stem cells (MSC): a comparison of adult and neonatal tissue-derived MSC. Cell Commun. Signal. 9, 12 (2011).
Prasanna, P. G. et al. Therapy-induced senescence: opportunities to improve anticancer therapy. J. Natl Cancer Inst. 113, 1285–1298 (2021).
Clark, K. C., Wu, Y., Taylor, R. A. & Daly, R. J. Novel therapeutic targets and biomarkers associated with prostate cancer-associated fibroblasts (CAFs). Crit. Rev. Oncog. 27, 1–24 (2022).
Bonollo, F., Thalmann, G. N., de Julio, M. K. & Karkampouna, S. The role of cancer-associated fibroblasts in prostate cancer tumorigenesis. Cancers 12, 1887 (2020).
Karin, O. & Alon, U. Senescent cell accumulation mechanisms inferred from parabiosis. GeroScience 43, 329–341 (2021).
Karin, O., Agrawal, A., Porat, Z., Krizhanovsky, V. & Alon, U. Senescent cell turnover slows with age providing an explanation for the Gompertz law. Nat. Commun. 10, 5495 (2019).
Hewitt, G. et al. Telomeres are favoured targets of a persistent DNA damage response in ageing and stress-induced senescence. Nat. Commun. 3, 708 (2012).
Shin, J.-S., Hong, A., Solomon, M. J. & Lee, C. S. The role of telomeres and telomerase in the pathology of human cancer and aging. Pathology 38, 103–113 (2006).
Deng, Y. & Chang, S. Role of telomeres and telomerase in genomic instability, senescence and cancer. Lab. Investig. 87, 1071–1076 (2007).
Crabbe, L., Jauch, A., Naeger, C. M., Holtgreve-Grez, H. & Karlseder, J. Telomere dysfunction as a cause of genomic instability in Werner syndrome. Proc. Natl Acad. Sci. USA 104, 2205–2210 (2007).
Tomasetti, C., Li, L. & Vogelstein, B. Stem cell divisions, somatic mutations, cancer etiology, and cancer prevention. Science 355, 1330–1334 (2017).
Chandeck, C. & Mooi, W. J. Oncogene-induced cellular senescence. Adv. Anat. Pathol. 17, 42–48 (2010).
Rattanavirotkul, N., Kirschner, K. & Chandra, T. Induction and transmission of oncogene-induced senescence. Cell. Mol. Life Sci. 78, 843–852 (2021).
Zhu, H. et al. Oncogene-induced senescence: from biology to therapy. Mech. Ageing Dev. 187, 111229 (2020).
Eng, C., Leone, G., Orloff, M. S. & Ostrowski, M. C. Genomic alterations in tumor stroma. Cancer Res. 69, 6759–6764 (2009).
Lisanti, M. P., Martinez-Outschoorn, U. E. & Sotgia, F. Oncogenes induce the cancer-associated fibroblast phenotype: metabolic symbiosis and “fibroblast addiction” are new therapeutic targets for drug discovery. Cell Cycle 12, 2723 (2013).
Chen, M. S., Lee, R. T. & Garbern, J. C. Senescence mechanisms and targets in the heart. Cardiovasc. Res. 118, 1173–1187 (2022).
Petrova, V., Annicchiarico-Petruzzelli, M., Melino, G. & Amelio, I. The hypoxic tumour microenvironment. Oncogenesis 7, 538–544 (2018).
Welford, S. M. & Giaccia, A. J. Hypoxia and senescence: the impact of oxygenation on tumor suppression. Mol. Cancer Res. 9, 538–544 (2011).
Usugi, E. et al. Antifibrotic agent pirfenidone suppresses proliferation of human pancreatic cancer cells by inducing G0/G1 cell cycle arrest. Pharmacology 103, 250–256 (2019).
Magi-Galluzzi, C., Sanderson, H. & Epstein, J. I. Atypia in nonneoplastic prostate glands after radiotherapy for prostate cancer: duration of atypia and relation to type of radiotherapy. Am. J. Surg. Pathol. 27, 206–212 (2003).
Sun, Y. et al. Treatment-induced damage to the tumor microenvironment promotes prostate cancer therapy resistance through WNT16B. Nat. Med. 18, 1359–1368 (2012). A genome-wide analysis of transcriptional responses to genotoxic stress shows that genotoxic cancer therapeutics induced transcriptional changes in the tumour microenvironment that promoted resistance to prostate cancer treatment.
Kim, J. H., Brown, S. L. & Gordon, M. N. Radiation-induced senescence: therapeutic opportunities. Radiat. Oncol. 18, 10 (2023).
Roediger, J. et al. Supraphysiological androgen levels induce cellular senescence in human prostate cancer cells through the Src-Akt pathway. Mol. Cancer 13, 214 (2014).
Hessenkemper, W. et al. A natural androgen receptor antagonist induces cellular senescence in prostate cancer cells. Mol. Endocrinol. 28, 1831–1840 (2014).
Roell, D. et al. Halogen-substituted anthranilic acid derivatives provide a novel chemical platform for androgen receptor antagonists. J. Steroid Biochem. Mol. Biol. 188, 59–70 (2019).
Fousteris, M. A. et al. 20-Aminosteroids as a novel class of selective and complete androgen receptor antagonists and inhibitors of prostate cancer cell growth. Bioorg. Med. Chem. 18, 6960–6969 (2010).
Mirochnik, Y. et al. Androgen receptor drives cellular senescence. PLoS ONE 7, e31052 (2012).
Pungsrinont, T. et al. Senolytic compounds control a distinct fate of androgen receptor agonist- and antagonist-induced cellular senescent LNCaP prostate cancer cells. Cell Biosci. 10, 59 (2020).
Palethorpe, H. M., Leach, D. A., Need, E. F., Drew, P. A. & Smith, E. Myofibroblast androgen receptor expression determines cell survival in co-cultures of myofibroblasts and prostate cancer cells in vitro. Oncotarget 9, 19100–19114 (2018).
Henshall, S. M. et al. Altered expression of androgen receptor in the malignant epithelium and adjacent stroma is associated with early relapse in prostate cancer. Cancer Res. 61, 423–427 (2001).
Cunha, G. R. et al. Hormonal, cellular, and molecular regulation of normal and neoplastic prostatic development. J. Steroid Biochem. Mol. Biol. 92, 221–236 (2004).
The Human Protein Atlas single cell type — prostate. The Human Protein Atlas https://www.proteinatlas.org/ENSG00000169083-AR/single+cell+type/prostate (2023).
Human normal and BPH prostate. Strand Lab https://strandlab.net/sc.data/pdpgb/ (2023).
Yue, Z. et al. Senescence-associated secretory phenotype and its impact on oral immune homeostasis. Front. Immunol. 13, 1019313 (2022).
Waters, D. W. et al. A senescence bystander effect in human lung fibroblasts. Biomedicines 9, 1162 (2021).
Nelson, G., Kucheryavenko, O., Wordsworth, J. & von Zglinicki, T. The senescent bystander effect is caused by ROS-activated NF-κB signalling. Mech. Ageing Dev. 170, 30–36 (2018).
Mikuła-Pietrasik, J. et al. Bystander senescence in human peritoneal mesothelium and fibroblasts is related to thrombospondin-1-dependent activation of transforming growth factor-β1. Int. J. Biochem. Cell Biol. 45, 2087–2096 (2013).
Nelson, G. et al. A senescent cell bystander effect: senescence-induced senescence. Aging Cell 11, 345–349 (2012).
Aw, D., Silva, A. B. & Palmer, D. B. Immunosenescence: emerging challenges for an ageing population. Immunology 120, 435–446 (2007).
Bodnar, A. G. et al. Extension of life-span by introduction of telomerase into normal human cells. Science 279, 349–352 (1998).
Göbel, A. et al. The role of inflammation in breast and prostate cancer metastasis to bone. Int. J. Mol. Sci. 22, 5078 (2021).
Tewari, A. K., Stockert, J. A., Yadav, S. S., Yadav, K. K. & Khan, I. Inflammation and prostate cancer. Adv. Exp. Med. Biol. 1095, 41–65 (2018).
Puhr, M. et al. Inflammation, microbiota, and prostate cancer. Eur. Urol. Focus 2, 374–382 (2016).
Sfanos, K. S., Yegnasubramanian, S., Nelson, W. G. & De Marzo, A. M. The inflammatory microenvironment and microbiome in prostate cancer development. Nat. Rev. Urol. 15, 11–24 (2018).
Stark, T., Livas, L. & Kyprianou, N. Inflammation in prostate cancer progression and therapeutic targeting. Transl. Androl. Urol. 4, 455–463 (2015).
Rani, A., Dasgupta, P. & Murphy, J. J. Prostate cancer: the role of inflammation and chemokines. Am. J. Pathol. 189, 2119–2137 (2019).
Kohli, K., Pillarisetty, V. G. & Kim, T. S. Key chemokines direct migration of immune cells in solid tumors. Cancer Gene Ther. 29, 10–21 (2022).
Kay, J., Thadhani, E., Samson, L. & Engelward, B. Inflammation-induced DNA damage, mutations and cancer. DNA Repair 83, 102673 (2019).
Papaconstantinou, J. The role of signaling pathways of inflammation and oxidative stress in development of senescence and aging phenotypes in cardiovascular disease. Cells 8, 1383 (2019).
Begley, L. A., Kasina, S., MacDonald, J. & Macoska, J. A. The inflammatory microenvironment of the aging prostate facilitates cellular proliferation and hypertrophy. Cytokine 43, 194–199 (2008).
Prata, L. G. P. L., Ovsyannikova, I. G., Tchkonia, T. & Kirkland, J. L. Senescent cell clearance by the immune system: emerging therapeutic opportunities. Semin. Immunol. 40, 101275 (2018).
Chen, S. et al. Single-cell analysis reveals transcriptomic remodellings in distinct cell types that contribute to human prostate cancer progression. Nat. Cell Biol. 23, 87–98 (2021).
Zhang, W. et al. The bone microenvironment invigorates metastatic seeds for further dissemination. Cell 184, 2471–2486.e20 (2021).
De Vivar, A. D. et al. Histologic features of stromogenic carcinoma of the prostate (carcinomas with reactive stroma grade 3). Hum. Pathol. 63, 202–211 (2017).
Turner, C. J. & Edwards, C. M. The role of the microenvironment in prostate cancer-associated bone disease. Curr. Osteoporos. Rep. 14, 170–177 (2016).
Gravina, G. L. et al. Phenotypic characterization of human prostatic stromal cells in primary cultures derived from human tissue samples. Int. J. Oncol. 42, 2116–2122 (2013).
Savant, S. S., Sriramkumar, S. & O’hagan, H. M. The role of inflammation and inflammatory mediators in the development, progression, metastasis, and chemoresistance of epithelial ovarian cancer. Cancers 10, 251 (2018).
Nguyen, D. P., Li, J. & Tewari, A. K. Inflammation and prostate cancer: the role of interleukin 6 (IL-6). BJU Int. 113, 986–992 (2014).
Culig, Z. Interleukin-6 function and targeting in prostate cancer. Adv. Exp. Med. Biol. 1290, 1–8 (2021).
Culig, Z. & Puhr, M. Interleukin-6 and prostate cancer: current developments and unsolved questions. Mol. Cell. Endocrinol. 462, 25–30 (2018).
Xu, H., Ding, Q. & Jiang, H. W. Genetic polymorphism of interleukin-1A (IL-1A), IL-1B, and IL-1 receptor antagonist (IL-1RN) and prostate cancer risk. Asian Pacif. J. Cancer Prev. 15, 8741–8747 (2014).
Graham, M. K. & Meeker, A. Telomeres and telomerase in prostate cancer development and therapy. Nat. Rev. Urol. 14, 607–619 (2017).
Heaphy, C. M. et al. Prostate stromal cell telomere shortening is associated with risk of prostate cancer in the placebo arm of the Prostate Cancer Prevention Trial. Prostate 75, 1160–1166 (2015).
Heaphy, C. M. et al. The prostate tissue-based telomere biomarker as a prognostic tool for metastasis and death from prostate cancer after prostatectomy. J. Pathol. Clin. Res. 8, 481–491 (2022).
Heaphy, C. M. et al. Prostate cancer cell telomere length variability and stromal cell telomere length as prognostic markers for metastasis and death. Cancer Discov. 3, 1130–1141 (2013). Stromal cell telomere shortening, one of the many markers of senescence, in combination with variable telomere lengths in prostate cancer cells, is a predictor of increased risk of death in men with prostate cancer.
De Leo, F. et al. Diflunisal targets the HMGB 1/ CXCL 12 heterocomplex and blocks immune cell recruitment. EMBO Rep. 20, e47788 (2019).
Li, X. et al. CXCL12/CXCR4 pathway orchestrates CSC-like properties by CAF recruited tumor associated macrophage in OSCC. Exp. Cell Res. 378, 131–138 (2019).
Liekens, S., Schols, D. & Hatse, S. CXCL12–CXCR4 axis in angiogenesis, metastasis and stem cell mobilization. Curr. Pharm. Des. 16, 3903–3920 (2011).
Susek, K. H., Karvouni, M., Alici, E. & Lundqvist, A. The role of CXC chemokine receptors 1–4 on immune cells in the tumor microenvironment. Front. Immunol. 9, 2159 (2018).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Gonzalez, H., Hagerling, C. & Werb, Z. Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes. Dev. 32, 1267–1284 (2018).
Waldman, A. D., Fritz, J. M. & Lenardo, M. J. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat. Rev. Immunol. 20, 651–668 (2020).
Wang, I. et al. Prostate cancer immunotherapy: a review of recent advancements with novel treatment methods and efficacy. Am. J. Clin. Exp. Urol. 10, 210–233 (2022).
Liu, Y. T. & Sun, Z. J. Turning cold tumors into hot tumors by improving T-cell infiltration. Theranostics 11, 5265–5286 (2021).
Galon, J. & Bruni, D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat. Rev. Drug. Discov. 18, 197–218 (2019).
Nair, S. S., Weil, R., Dovey, Z., Davis, A. & Tewari, A. K. The tumor microenvironment and immunotherapy in prostate and bladder cancer. Urol. Clin. North Am. 47, e17–e54 (2020).
Peng, S. et al. Single-cell analysis reveals EP4 as a target for restoring T-cell infiltration and sensitizing prostate cancer to immunotherapy. Clin. Cancer Res. 28, 552–567 (2022).
Wallace, T. A. et al. Tumor immunobiological differences in prostate cancer between African-American and European-American men. Cancer Res. 68, 927–936 (2008).
Tang, W. et al. IFNL4-ΔG allele is associated with an interferon signature in tumors and survival of African-American men with prostate cancer. Clin. Cancer Res. 24, 5471–5481 (2018).
Rayford, W. et al. Comparative analysis of 1152 African-American and European-American men with prostate cancer identifies distinct genomic and immunological differences. Commun. Biol. 4, 670 (2021). A retrospective analysis of patients with suspected prostate cancer who underwent radical prostatectomy shows that African American men have an enriched immune signature compared with European American men.
Kiely, M. & Ambs, S. Immune inflammation pathways as therapeutic targets to reduce lethal prostate cancer in African American men. Cancers 13, 2874 (2021).
Awasthi, S. et al. Comparative genomics reveals distinct immune-oncologic pathways in African American men with prostate cancer. Clin. Cancer Res. 27, 320–329 (2021).
Batai, K., Murphy, A. B., Nonn, L. & Kittles, R. A. Vitamin D and immune response: implications for prostate cancer in African Americans. Front. Immunol. 7, 53 (2016).
Gillard, M. et al. Elevation of stromal-derived mediators of inflammation promote prostate cancer progression in African-American men. Cancer Res. 78, 6134–6145 (2018).
Smith, C. J. et al. Aspirin use reduces the risk of aggressive prostate cancer and disease recurrence in African-American men. Cancer Epidemiol. Biomark. Prev. 26, 845–853 (2017).
Sartor, O. et al. Survival of African-American and Caucasian men after sipuleucel-T immunotherapy: outcomes from the PROCEED registry. Prostate Cancer Prostatic Dis. 23, 517–526 (2020).
Kirkland, J. L. & Tchkonia, T. Cellular senescence: a translational perspective. eBioMedicine 21, 21–28 (2017).
Scudellari, M. To stay young, kill zombies. Nature 550, 448–450 (2017).
Chaib, S., Tchkonia, T. & Kirkland, J. L. Cellular senescence and senolytics: the path to the clinic. Nat. Med. 28, 1556–1568 (2022).
Raffaele, M. & Vinciguerra, M. The costs and benefits of senotherapeutics for human health. Lancet Healthy Longev. 3, e67–e77 (2022).
Kim, E. C. & Kim, J. R. Senotherapeutics: emerging strategy for healthy aging and age-related disease. BMB Rep. 52, 47–55 (2019).
Choi, J. Y., Yee, S. F., Tchangalova, T., Yang, G. & Fisher, J. P. Recent advances in senotherapeutics delivery. Tissue Eng. B 28, 1223–1234 (2022).
Zhang, L., Pitcher, L. E., Prahalad, V., Niedernhofer, L. J. & Robbins, P. D. Targeting cellular senescence with senotherapeutics: senolytics and senomorphics. FEBS J. 290, 1362–1383 (2023).
Sánchez-Díaz, L., Espinosa-Sánchez, A., Blanco, J. R. & Carnero, A. Senotherapeutics in cancer and HIV. Cells 11, 1222 (2022).
Schwarze, S. R., Fu, V. X., Desotelle, J. A., Kenowski, M. L. & Jarrard, D. F. The identification of senescence-specific genes during the induction of senescence in prostate cancer cells. Neoplasia 7, 816–823 (2005).
Macoska, J. A. et al. Pilot and feasibility study of serum chemokines as markers to distinguish prostatic disease in men with low total serum PSA. Prostate 68, 442–452 (2008).
Jochems, F. et al. The Cancer SENESCopedia: a delineation of cancer cell senescence. Cell Rep. 36, 109441 (2021).
Begley, L. A., MacDonald, J. W., Day, M. L. & Macoska, J. A. CXCL12 activates a robust transcriptional response in human prostate epithelial cells. J. Biol. Chem. 282, 26767–26774 (2007).
Mylonas, K. J. et al. Cellular senescence inhibits renal regeneration after injury in mice, with senolytic treatment promoting repair. Sci. Transl. Med. 13, eabb0203 (2021).
Raffaele, M. et al. Mild exacerbation of obesity- and age-dependent liver disease progression by senolytic cocktail dasatinib+quercetin. Cell Commun. Signal. 19, 44 (2021).
Peng, X. et al. Cellular senescence contributes to radiation-induced hyposalivation by affecting the stem/progenitor cell niche. Cell Death Dis. 11, 854 (2020).
Alessio, N. et al. Biomolecular evaluation of piceatannol’s effects in counteracting the senescence of mesenchymal stromal cells: a new candidate for senotherapeutics? Int. J. Mol. Sci. 22, 11619 (2021). An in vitro evaluation of piceatannol demonstrates its selective senotherapeutic potential against senescent mesenchymal stromal cells.
Lewinska, A. et al. AMPK-mediated senolytic and senostatic activity of quercetin surface functionalized Fe3O4 nanoparticles during oxidant-induced senescence in human fibroblasts. Redox Biol. 28, 101337 (2020). Natural compounds and repurposed drugs with senolytics and senomorphic activities.
Palacio, L. et al. Restored immune cell functions upon clearance of senescence in the irradiated splenic environment. Aging Cell 18, e12971 (2019).
Garrido, A. M. et al. Efficacy and limitations of senolysis in atherosclerosis. Cardiovasc. Res. 118, 1713–1727 (2022).
Owens, W. A., Walaszczyk, A., Spyridopoulos, I., Dookun, E. & Richardson, G. D. Senescence and senolytics in cardiovascular disease: promise and potential pitfalls. Mech. Ageing Dev. 198, 111540 (2021).
Boccardi, V. & Mecocci, P. Senotherapeutics: targeting senescent cells for the main age-related diseases. Mech. Ageing Dev. 197, 111526 (2021).
Carpenter, V. et al. Androgen-deprivation induced senescence in prostate cancer cells is permissive for the development of castration-resistance but susceptible to senolytic therapy. Biochem. Pharmacol. 193, 114765 (2021).
Malaquin, N. et al. DNA damage- but not enzalutamide-induced senescence in prostate cancer promotes senolytic Bcl-xL inhibitor sensitivity. Cells 9, 1593 (2020).
Peng, Y. et al. Ginsenoside Rg3 inhibits the senescence of prostate stromal cells through down-regulation of interleukin 8 expression. Oncotarget 8, 64779–64792 (2017).
El Maaty, M. A. A. et al. Single-cell analyses unravel cell type-specific responses to a vitamin D analog in prostatic precancerous lesions. Sci. Adv. 7, eabg5982 (2021).
Liu, H. et al. Rutin is a potent senomorphic agent to target senescent cells and can improve chemotherapeutic efficacy. Aging Cell https://doi.org/10.1111/acel.13921 (2023).
Baghban, R. et al. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun. Signal. 18, 59 (2020).
Wu, Y., Song, Y., Wang, R. & Wang, T. Molecular mechanisms of tumor resistance to radiotherapy. Mol. Cancer 22, 96 (2023).
Ngoi, N. Y. et al. The redox–senescence axis and its therapeutic targeting. Redox Biol. 45, 102032 (2021).
Lall, R. K., Adhami, V. M. & Mukhtar, H. Dietary flavonoid fisetin for cancer prevention and treatment. Mol. Nutr. Food Res. 60, 1396–1405 (2016).
Li, W., He, Y., Zhang, R., Zheng, G. & Zhou, D. The curcumin analog EF24 is a novel senolytic agent. Aging 11, 771–782 (2019).
Kale, A. et al. Role of immune cells in the removal of deleterious senescent cells. Immun. Ageing 17, 16 (2020).
Demaria, M. et al. Cellular senescence promotes adverse effects of chemotherapy and cancer relapse. Cancer Discov. 7, 165–176 (2017).
Larionova, I. et al. Tumor-associated macrophages in human breast, colorectal, lung, ovarian and prostate cancers. Front. Oncol. 10, 566511 (2020).
Boibessot, C. & Toren, P. Sex steroids in the tumor microenvironment and prostate cancer progression. Endocr. Relat. Cancer 25, R179–R196 (2018).
Ge, R., Wang, Z. & Cheng, L. Tumor microenvironment heterogeneity an important mediator of prostate cancer progression and therapeutic resistance. npj Precis. Oncol. 6, 31 (2022).
Akoto, T. & Saini, S. Role of exosomes in prostate cancer metastasis. Int. J. Mol. Sci. 22, 3528 (2021).
Hofbauer, L. C. et al. Novel approaches to target the microenvironment of bone metastasis. Nat. Rev. Clin. Oncol. 18, 488–505 (2021).
Acknowledgements
E.A.P. and A.K.M. received support for this research from the National Cancer Institute (R01CA255349) and the Department of Defense Prostate Cancer Research Program (W81XWH-20-1-0264). W.N.B. received support for the research of this work from the National Cancer Institute (R01CA255259). A.M.D.M. received support for the research of this work from the National Cancer Institute (U01CA196390). S.Y., A.M.D.M. and W.N.B. received support for the research of this work from the National Cancer Institute (U54CA274370). S.R.D. received support for the research of this work from the National Cancer Institute (P50CA058236). The content of this work is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
J.O.M., I.E. and C.M.H. researched data for the article. J.O.M., W.N.B., M.K.G., T.L.L., A.M.D.M., S.R.D., S.Y., W.G.N., G.V.D., E.A.P., A.K.M., C.C.Y. and C.M.H. contributed substantially to discussion of the content. J.O.M., I.E., W.N.B., M.K.G., E.A.P., A.K.M. and C.M.H. wrote the article. All authors reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
T.L.L. has received research support from Roche/Ventana, AIRA Matrix, DeepBio and Myriad Genetics for other studies. C.C.Y. received honorarium or consultant fees from PreludeDx, QED Therapeutics, Amgen, Regeneron and Riptide Bioscience. C.C.Y. is a shareholder in Riptide Bioscience. A.M.D.M. has served as a Consultant for Merck and Cepheid and has received research grant funding from Janssen R&D. S.R.D. has received research funding for his institution from Astellas and Bayer. S.Y. has received grant support to his institution from Janssen, Bristol Myers Squibb and Cepheid. He has also previously served as a consultant to Cepheid. W.G.N. is a member of the Scientific Advisory Board for Cepheid and has received funding support from Bristol Myers Squibb. G.V.D. is a member of the scientific advisory board of Vyne Therapeutics. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Urology thanks Yu Sun and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mori, J.O., Elhussin, I., Brennen, W.N. et al. Prognostic and therapeutic potential of senescent stromal fibroblasts in prostate cancer. Nat Rev Urol (2023). https://doi.org/10.1038/s41585-023-00827-x
Accepted:
Published:
DOI: https://doi.org/10.1038/s41585-023-00827-x