Abstract
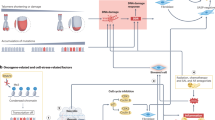
Senescent cells accumulate with age in all tissues. Although senescent cells undergo cell-cycle arrest, these cells remain metabolically active and their secretome — known as the senescence-associated secretory phenotype — is responsible for a systemic pro-inflammatory state, which contributes to an inflammatory microenvironment. Senescent cells can be found in the ageing prostate and the senescence-associated secretory phenotype and can be linked to BPH and prostate cancer. Indeed, a number of signalling pathways provide biological plausibility for the role of senescence in both BPH and prostate cancer, although proving causality is difficult. The theory of senescence as a mechanism for prostate disease has a number of clinical implications and could offer opportunities for targeting in the future.
Key points
-
BPH and prostate cancer are two common disorders affecting the prostate and both share an increased incidence with advancing age.
-
Ageing, along with other sources of cellular damage (infection, toxins, chemical or physical injury), results in cellular senescence and the accumulation of senescent cells in tissues.
-
Senescent cells, although unable to replicate, remain metabolically active and secrete a raft of inflammatory mediators, known as the senescence-associated secretory phenotype (SASP).
-
Senescent cells have been detected using senescence markers in almost all human samples of BPH, and the role of several components of the SASP has been established in BPH initiation and progression.
-
The role of cellular senescence in prostate cancer is less clearly established, and senescence seems to act mainly through the influence of the senescent stroma on adjacent epithelial cells, favouring cancer initiation, progression and metastasis.
-
The demonstration of the role of senescence in both age-related prostatic diseases presents new therapeutic opportunities with treatments aimed at removing senescent cells (senolytics) and/or targeting components of the SASP (SASP inhibitors or senomorphics).
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Berry, S. J., Coffey, D. S., Walsh, P. C. & Ewing, L. L. The development of human benign prostatic hyperplasia with age. J. Urol. 132, 474–479 (1984).
Soos, G. et al. The prevalence of prostate carcinoma and its precursor in Hungary: an autopsy study. Eur. Urol. 48, 739–744 (2005).
Bell, K. J. L., Del Mar, C., Wright, G., Dickinson, J. & Glasziou, P. Prevalence of incidental prostate cancer: A systematic review of autopsy studies. Int. J. Cancer 137, 1749–1757 (2015).
McNeal, J. E. The zonal anatomy of the prostate. Prostate 2, 35–49 (1981).
McNeal, J. E., Redwine, E. A., Freiha, F. S. & Stamey, T. A. Zonal distribution of prostatic adenocarcinoma. Correlation with histologic pattern and direction of spread. Am. J. Surg. Pathol. 12, 897–906 (1988).
Turkbey, B. et al. Age-related changes in prostate zonal volumes as measured by high-resolution magnetic resonance imaging (MRI): a cross-sectional study in over 500 patients. BJU Int. 110, 1642–1647 (2012).
De Nunzio, C. et al. The controversial relationship between benign prostatic hyperplasia and prostate cancer: the role of inflammation. Eur. Urol. 60, 106–117 (2011).
Cai, T. et al. Current knowledge of the potential links between inflammation and prostate cancer. Int. J. Mol. Sci. 20, 3833 (2019).
van Deursen, J. M. The role of senescent cells in ageing. Nature 509, 439–446 (2014).
Hayflick, L. & Moorhead, P. S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 25, 585–621 (1961).
Coppé, J.-P., Desprez, P.-Y., Krtolica, A. & Campisi, J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu. Rev. Pathol. 5, 99–118 (2010).
Campisi, J. & d’Adda di Fagagna, F. Cellular senescence: when bad things happen to good cells. Nat. Rev. Mol. Cell Biol. 8, 729–740 (2007).
Franceschi, C. & Campisi, J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. A. Biol. Sci. Med. Sci. 69 (Suppl. 1), S4–S9 (2014).
Huda, N. et al. Hepatic senescence, the good and the bad. World J. Gastroenterol. 25, 5069–5081 (2019).
Xu, M. et al. Transplanted senescent cells induce an osteoarthritis-like condition in mice. J. Gerontol. A. Biol. Sci. Med. Sci. 72, 780–785 (2017).
Baker, D. J. et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479, 232–236 (2011).
Xu, M. et al. Senolytics improve physical function and increase lifespan in old age. Nat. Med. 24, 1246–1256 (2018).
Roos, C. M. et al. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell 15, 973–977 (2016).
Yousefzadeh, M. J. et al. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine 36, 18–28 (2018).
Adamus, J., Aho, S., Meldrum, H., Bosko, C. & Lee, J.-M. p16INK4A influences the aging phenotype in the living skin equivalent. J. Invest. Dermatol. 134, 1131–1133 (2014).
Waaijer, M. E. C. et al. The number of p16INK4a positive cells in human skin reflects biological age. Aging Cell 11, 722–725 (2012).
Dimri, G. P. et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl Acad. Sci. USA 92, 9363–9367 (1995).
Smith, J. R. & Pereira-Smith, O. M. Replicative senescence: implications for in vivo aging and tumor suppression. Science 273, 63–67 (1996).
Byun, H.-O. et al. Cathepsin D and eukaryotic translation elongation factor 1 as promising markers of cellular senescence. Cancer Res. 69, 4638–4647 (2009).
Pruitt, F. L. et al. Cathepsin D acts as an essential mediator to promote malignancy of benign prostatic epithelium. Prostate 73, 476–488 (2013).
Kuilman, T., Michaloglou, C., Mooi, W. J. & Peeper, D. S. The essence of senescence. Genes Dev. 24, 2463–2479 (2010).
Biran, A. et al. Quantitative identification of senescent cells in aging and disease. Aging Cell 16, 661–671 (2017).
Childs, B. G., Bussian, T. J. & Baker, D. J. Cellular Identification and quantification of senescence-associated β-galactosidase activity in vivo. Methods Mol. Biol. 1896, 31–38 (2019).
Pereira, B. I. et al. Senescent cells evade immune clearance via HLA-E-mediated NK and CD8+ T cell inhibition. Nat. Commun. 10, 2387 (2019).
Schwarze, S. R., Fu, V. X., Desotelle, J. A., Kenowski, M. L. & Jarrard, D. F. The identification of senescence-specific genes during the induction of senescence in prostate cancer cells. Neoplasia 7, 816–823 (2005).
Halvorsen, O. J., Haukaas, S., Høisæter, P. Å. & Akslen, L. A. Expression of p 16 protein in prostatic adenocarcinomas, intraepithelial neoplasia, and benign/hyperplastic glands. Urol. Oncol. 3, 59–66 (1997).
Zhang, Z., Rosen, D. G., Yao, J. L., Huang, J. & Liu, J. Expression of p14ARF, p15INK4b, p16INK4a, and DCR2 increases during prostate cancer progression. Mod. Pathol. 19, 1339–1343 (2006).
Chen, Z. et al. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature 436, 725–730 (2005).
Alimonti, A. et al. A novel type of cellular senescence that can be enhanced in mouse models and human tumor xenografts to suppress prostate tumorigenesis. J. Clin. Invest. 120, 681–693 (2010).
Pernicová, Z. et al. Androgen depletion induces senescence in prostate cancer cells through down-regulation of Skp2. Neoplasia 13, 526–536 (2011).
Ewald, J. A. et al. Androgen deprivation induces senescence characteristics in prostate cancer cells in vitro and in vivo. Prostate 73, 337–345 (2013).
Parisotto, M. et al. PTEN deletion in luminal cells of mature prostate induces replication stress and senescence in vivo. J. Exp. Med. 215, 1749–1763 (2018).
Hensley, P. J. & Kyprianou, N. Modeling prostate cancer in mice: limitations and opportunities. J. Androl. 33, 133–144 (2012).
Collado, M. & Serrano, M. Senescence in tumours: evidence from mice and humans. Nat. Rev. Cancer 10, 51–57 (2010).
Oliveira, D. S. M. et al. The mouse prostate: a basic anatomical and histological guideline. Bosn. J. Basic. Med. Sci. 16, 8–13 (2016).
Choi, J. et al. Expression of senescence-associated beta-galactosidase in enlarged prostates from men with benign prostatic hyperplasia. Urology 56, 160–166 (2000).
Castro, P., Giri, D., Lamb, D. & Ittmann, M. Cellular senescence in the pathogenesis of benign prostatic hyperplasia. Prostate 55, 30–38 (2003).
Vital, P., Castro, P., Tsang, S. & Ittmann, M. The senescence-associated secretory phenotype promotes benign prostatic hyperplasia. Am. J. Pathol. 184, 721–731 (2014).
Jiang, S., Song, C. S. & Chatterjee, B. Stimulation of prostate cells by the senescence phenotype of epithelial and stromal cells: implication for benign prostate hyperpalsia. FASEB BioAdvances 1, 353–363 (2019).
Shapiro, E., Becich, M. J., Hartanto, V. & Lepor, H. The relative proportion of stromal and epithelial hyperplasia is related to the development of symptomatic benign prostate hyperplasia. J. Urol. 147, 1293–1297 (1992).
Vernier, M. et al. Regulation of E2Fs and senescence by PML nuclear bodies. Genes Dev. 25, 41–50 (2011).
Deschênes-Simard, X. et al. Tumor suppressor activity of the ERK/MAPK pathway by promoting selective protein degradation. Genes Dev. 27, 900–915 (2013).
Krtolica, A. & Campisi, J. Integrating epithelial cancer, aging stroma and cellular senescence. Adv. Gerontol. 11, 109–116 (2003).
McNeal, J. E. Origin and evolution of benign prostatic enlargement. Invest. Urol. 15, 340–345 (1978).
Untergasser, G., Madersbacher, S. & Berger, P. Benign prostatic hyperplasia: age-related tissue-remodeling. Exp. Gerontol. 40, 121–128 (2005).
Bierhoff, E. et al. Morphological analogies of fetal prostate stroma and stromal nodules in BPH. Prostate 31, 234–240 (1997).
Cunha, G. R. & Ricke, W. A. A historical perspective on the role of stroma in the pathogenesis of benign prostatic hyperplasia. Differ. Res. Biol. Divers. 82, 168–172 (2011).
Muñoz-Espín, D. et al. Programmed cell senescence during mammalian embryonic development. Cell 155, 1104–1118 (2013).
Felka, T. et al. Loss of spatial organization and destruction of the pericellular matrix in early osteoarthritis in vivo and in a novel in vitro methodology. Osteoarthritis Cartilage 24, 1200–1209 (2016).
Price, J. S. et al. The role of chondrocyte senescence in osteoarthritis. Aging Cell 1, 57–65 (2002).
Childs, B. G. et al. Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science 354, 472–477 (2016).
Harman, S. M. et al. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J. Clin. Endocrinol. Metab. 86, 724–731 (2001).
Roehrborn, C. G., Marks, L. & Harkaway, R. Enlarged prostate: a landmark national survey of its prevalence and impact on US men and their partners. Prostate Cancer Prostatic Dis. 9, 30–34 (2006).
Thompson, I. M. et al. The influence of finasteride on the development of prostate cancer. N. Engl. J. Med. 349, 215–224 (2003).
Kristal, A. R. et al. Serum steroid and sex hormone-binding globulin concentrations and the risk of incident benign prostatic hyperplasia: results from the prostate cancer prevention trial. Am. J. Epidemiol. 168, 1416–1424 (2008).
Boyle, P. et al. Endogenous and exogenous testosterone and the risk of prostate cancer and increased prostate-specific antigen (PSA) level: a meta-analysis. BJU Int. 118, 731–741 (2016).
Mohamad, N.-V. et al. The relationship between circulating testosterone and inflammatory cytokines in men. Aging Male 22, 129–140 (2019).
Chen, Y.-Q. et al. Testosterone ameliorates vascular aging via the Gas6/Axl signaling pathway. Aging 12, 16111–16125 (2020).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
De Nunzio, C., Presicce, F. & Tubaro, A. Inflammatory mediators in the development and progression of benign prostatic hyperplasia. Nat. Rev. Urol. 13, 613–626 (2016).
Nickel, J. C. et al. Consensus development of a histopathological classification system for chronic prostatic inflammation. BJU Int. 87, 797–805 (2001).
Nickel, J. C. Prostatitis. Can. Urol. Assoc. J. 5, 306–315 (2011).
Sfanos, K. S., Yegnasubramanian, S., Nelson, W. G. & De Marzo, A. M. The inflammatory microenvironment and microbiome in prostate cancer development. Nat. Rev. Urol. 15, 11–24 (2018).
Robert, G. et al. Inflammation in benign prostatic hyperplasia: a 282 patients’ immunohistochemical analysis. Prostate 69, 1774–1780 (2009).
Delongchamps, N. B. et al. Evaluation of prostatitis in autopsied prostates — is chronic inflammation more associated with benign prostatic hyperplasia or cancer? J. Urol. 179, 1736–1740 (2008).
McConnell, J. D. et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N. Engl. J. Med. 349, 2387–2398 (2003).
Torkko, K. C. et al. Prostate biopsy markers of inflammation are associated with risk of clinical progression of benign prostatic hyperplasia: findings from the MTOPS study. J. Urol. 194, 454–461 (2015).
Vesalainen, S., Lipponen, P., Talja, M. & Syrjänen, K. Histological grade, perineural infiltration, tumour-infiltrating lymphocytes and apoptosis as determinants of long-term prognosis in prostatic adenocarcinoma. Eur. J. Cancer 30, 1797–1803 (1994).
Irani, J. et al. High-grade inflammation in prostate cancer as a prognostic factor for biochemical recurrence after radical prostatectomy. Urology 54, 467–472 (1999).
Rani, A., Dasgupta, P. & Murphy, J. J. Prostate cancer: the role of inflammation and chemokines. Am. J. Pathol. 189, 2119–2137 (2019).
Sfanos, K. S., Yegnasubramanian, S., Nelson, W. G. & De Marzo, A. M. The inflammatory microenvironment and microbiome in prostate cancer development. Nat. Rev. Urol. 15, 11–24 (2017).
De Marzo, A. M. et al. Inflammation in prostate carcinogenesis. Nat. Rev. Cancer 7, 256–269 (2007).
De Marzo, A. M., Haffner, M. C., Lotan, T. L., Yegnasubramanian, S. & Nelson, W. G. Premalignancy in prostate cancer: rethinking what we know. Cancer Prev. Res. 9, 648–656 (2016).
Gerrin, S. J., Sowalsky, A. G., Balk, S. P. & Ye, H. Mutation profiling indicates high grade prostatic intraepithelial neoplasia as distant precursors of adjacent invasive prostatic adenocarcinoma: mutation profile of HGPIN. Prostate 76, 1227–1236 (2016).
Toso, A. et al. Enhancing chemotherapy efficacy in Pten-deficient prostate tumors by activating the senescence-associated antitumor immunity. Cell Rep. 9, 75–89 (2014).
Kaur, H. B. et al. Association of tumor-infiltrating T-cell density with molecular subtype, racial ancestry and clinical outcomes in prostate cancer. Mod. Pathol. 31, 1539–1552 (2018).
Kaur, H. B. et al. TP53 missense mutation is associated with increased tumor-infiltrating T cells in primary prostate cancer. Hum. Pathol. 87, 95–102 (2019).
Patarroyo, M., Tryggvason, K. & Virtanen, I. Laminin isoforms in tumor invasion, angiogenesis and metastasis. Semin. Cancer Biol. 12, 197–207 (2002).
Sprenger, C. C. T. et al. Senescence-induced alterations of laminin chain expression modulate tumorigenicity of prostate cancer cells. Neoplasia 10, 1350–1361 (2008).
Platz, E. A. et al. A prospective study of chronic inflammation in benign prostate tissue and risk of prostate cancer: linked PCPT and SELECT cohorts. Cancer Epidemiol. Biomarkers Prev. 26, 1549–1557 (2017).
Jiang, J. et al. The role of prostatitis in prostate cancer: meta-analysis. PLoS ONE 8, e85179 (2013).
Langston, M. E. et al. A systematic review and meta-analysis of associations between clinical prostatitis and prostate cancer: new estimates accounting for detection bias. Cancer Epidemiol. Biomarkers Prev. 28, 1594–1603 (2019).
de Bono, J. S. et al. Prostate carcinogenesis: inflammatory storms. Nat. Rev. Cancer 20, 455–469 (2020).
Shinohara, D. B. et al. A mouse model of chronic prostatic inflammation using a human prostate cancer-derived isolate of Propionibacterium acnes. Prostate 73, 1007–1015 (2013).
Kirby, R. S., Lowe, D., Bultitude, M. I. & Shuttleworth, K. E. Intra-prostatic urinary reflux: an aetiological factor in abacterial prostatitis. Br. J. Urol. 54, 729–731 (1982).
Liu, C., La Rosa, S. & Hagos, E. G. Oxidative DNA damage causes premature senescence in mouse embryonic fibroblasts deficient for Krüppel-like factor 4. Mol. Carcinog. 54, 889–899 (2015).
Wang, B., Kohli, J. & Demaria, M. Senescent cells in cancer therapy: friends or foes? Trends Cancer 6, 838–857 (2020).
Nguyen, D. P., Li, J. & Tewari, A. K. Inflammation and prostate cancer: the role of interleukin 6 (IL-6). BJU Int. 113, 986–992 (2014).
Shariat, S. F. et al. Plasma levels of interleukin-6 and its soluble receptor are associated with prostate cancer progression and metastasis. Urology 58, 1008–1015 (2001).
Maynard, J. P. et al. IL8 expression is associated with prostate cancer aggressiveness and androgen receptor loss in primary and metastatic prostate cancer. Mol. Cancer Res. 18, 153–165 (2020).
Giri, D. & Ittmann, M. Interleukin-1alpha is a paracrine inducer of FGF7, a key epithelial growth factor in benign prostatic hyperplasia. Am. J. Pathol. 157, 249–255 (2000).
Giri, D. & Ittmann, M. Interleukin-8 is a paracrine inducer of fibroblast growth factor 2, a stromal and epithelial growth factor in benign prostatic hyperplasia. Am. J. Pathol. 159, 139–147 (2001).
Castro, P., Xia, C., Gomez, L., Lamb, D. J. & Ittmann, M. Interleukin-8 expression is increased in senescent prostatic epithelial cells and promotes the development of benign prostatic hyperplasia. Prostate 60, 153–159 (2004).
Tominaga, K. & Suzuki, H. I. TGF-β signaling in cellular senescence and aging-related pathology. Int. J. Mol. Sci. 20, 5002 (2019).
Royuela, M. et al. Transforming growth factor beta 1 and its receptor types I and II. Comparison in human normal prostate, benign prostatic hyperplasia, and prostatic carcinoma. Growth Factors 16, 101–110 (1998).
Untergasser, G. et al. Profiling molecular targets of TGF-beta1 in prostate fibroblast-to-myofibroblast transdifferentiation. Mech. Ageing Dev. 126, 59–69 (2005).
Walenda, G. et al. TGF-beta1 does not induce senescence of multipotent mesenchymal stromal cells and has similar effects in early and late passages. PLoS ONE 8, e77656 (2013).
Wang, L. et al. Aberrant transforming growth factor-β activation recruits mesenchymal stem cells during prostatic hyperplasia. Stem Cell Transl Med. 6, 394–404 (2017).
Wang, R. et al. Long noncoding RNA DNM3OS promotes prostate stromal cells transformation via the miR-29a/29b/COL3A1 and miR-361/TGFβ1 axes. Aging 11, 9442–9460 (2019).
Hu, S. et al. Evidence of TGF-β1 mediated epithelial-mesenchymal transition in immortalized benign prostatic hyperplasia cells. Mol. Membr. Biol. 31, 103–110 (2014).
Elliott, R. L. & Blobe, G. C. Role of transforming growth factor beta in human cancer. J. Clin. Oncol. 23, 2078–2093 (2005).
Tuxhorn, J. A. et al. Reactive stroma in human prostate cancer: induction of myofibroblast phenotype and extracellular matrix remodeling. Clin. Cancer Res. 8, 2912–2923 (2002).
Zhang, H. et al. lncRNA MIR4435-2HG promotes cancer cell migration and invasion in prostate carcinoma by upregulating TGF-β1. Oncol. Lett. 18, 4016–4021 (2019).
Paller, C. et al. TGF-β receptor I inhibitor enhances response to enzalutamide in a pre-clinical model of advanced prostate cancer. Prostate 79, 31–43 (2019).
Romero, D. et al. Dickkopf-3 regulates prostate epithelial cell acinar morphogenesis and prostate cancer cell invasion by limiting TGF-β-dependent activation of matrix metalloproteases. Carcinogenesis 37, 18–29 (2016).
Begley, L., Monteleon, C., Shah, R. B., Macdonald, J. W. & Macoska, J. A. CXCL12 overexpression and secretion by aging fibroblasts enhance human prostate epithelial proliferation in vitro. Aging Cell 4, 291–298 (2005).
Bavik, C. et al. The gene expression program of prostate fibroblast senescence modulates neoplastic epithelial cell proliferation through paracrine mechanisms. Cancer Res. 66, 794–802 (2006).
Ao, M. et al. Cross-talk between paracrine-acting cytokine and chemokine pathways promotes malignancy in benign human prostatic epithelium. Cancer Res. 67, 4244–4253 (2007).
Linxweiler, J. & Junker, K. Extracellular vesicles in urological malignancies: an update. Nat. Rev. Urol. 17, 11–27 (2020).
Junker, K., Heinzelmann, J., Beckham, C., Ochiya, T. & Jenster, G. Extracellular vesicles and their role in urologic malignancies. Eur. Urol. 70, 323–331 (2016).
Valentino, A. et al. Exosomal microRNAs in liquid biopsies: future biomarkers for prostate cancer. Clin. Transl Oncol. 19, 651–657 (2017).
Urbanelli, L., Buratta, S., Sagini, K., Tancini, B. & Emiliani, C. Extracellular vesicles as new players in cellular senescence. Int. J. Mol. Sci. 17, 1408 (2016).
Jakhar, R. & Crasta, K. Exosomes as emerging pro-tumorigenic mediators of the senescence-associated secretory phenotype. Int. J. Mol. Sci. 20, 2547 (2019).
Lehmann, B. D. et al. Senescence-associated exosome release from human prostate cancer cells. Cancer Res. 68, 7864–7871 (2008).
Alibhai, F. J. et al. Cellular senescence contributes to age-dependent changes in circulating extracellular vesicle cargo and function. Aging Cell 19, e13103 (2020).
Yaman Agaoglu, F. et al. Investigation of miR-21, miR-141, and miR-221 in blood circulation of patients with prostate cancer. Tumour Biol. 32, 583–588 (2011).
Foj, L. et al. Exosomal and non-exosomal urinary miRNAs in prostate cancer detection and prognosis. Prostate 77, 573–583 (2017).
Elkhattouti, A., Hassan, M. & Gomez, C. R. Stromal fibroblast in age-related cancer: role in tumorigenesis and potential as novel therapeutic target. Front. Oncol. 5, 158 (2015).
Giannoni, E. et al. Reciprocal activation of prostate cancer cells and cancer-associated fibroblasts stimulates epithelial-mesenchymal transition and cancer stemness. Cancer Res. 70, 6945–6956 (2010).
Sun, D.-Y., Wu, J.-Q., He, Z.-H., He, M.-F. & Sun, H.-B. Cancer-associated fibroblast regulate proliferation and migration of prostate cancer cells through TGF-β signaling pathway. Life Sci. 235, 116791 (2019).
Vickman, R. E. et al. Heterogeneity of human prostate carcinoma-associated fibroblasts implicates a role for subpopulations in myeloid cell recruitment. Prostate 80, 173–185 (2020).
Liu, Y. et al. Klotho-mediated targeting of CCL2 suppresses the induction of colorectal cancer progression by stromal cell senescent microenvironments. Mol. Oncol. 13, 2460–2475 (2019).
Jin, B. et al. PIM-1 modulates cellular senescence and links IL-6 signaling to heterochromatin formation. Aging Cell 13, 879–889 (2014).
Deschênes-Simard, X., Kottakis, F., Meloche, S. & Ferbeyre, G. ERKs in cancer: friends or foes? Cancer Res. 74, 412–419 (2014).
van Deursen, J. M. Senolytic therapies for healthy longevity. Science 364, 636–637 (2019).
Vukmanovic-Stejic, M. et al. Enhancement of cutaneous immunity during aging by blocking p38 mitogen-activated protein (MAP) kinase-induced inflammation. J. Allergy Clin. Immunol. 142, 844–856 (2018).
von Kobbe, C. Targeting senescent cells: approaches, opportunities, challenges. Aging 11, 12844–12861 (2019).
Chen, X. & Song, E. Turning foes to friends: targeting cancer-associated fibroblasts. Nat. Rev. Drug Discov. 18, 99–115 (2019).
Laberge, R.-M. et al. MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nat. Cell Biol. 17, 1049–1061 (2015).
Fung, A. S., Wu, L. & Tannock, I. F. Concurrent and sequential administration of chemotherapy and the mammalian target of rapamycin inhibitor temsirolimus in human cancer cells and xenografts. Clin. Cancer Res. 15, 5389–5395 (2009).
Sprott, R. L. Biomarkers of aging and disease: introduction and definitions. Exp. Gerontol. 45, 2–4 (2010).
Coppé, J.-P. et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 6, 2853–2868 (2008).
Brennen, W. N. & Isaacs, J. T. Mesenchymal stem cells and the embryonic reawakening theory of BPH. Nat. Rev. Urol. 15, 703–715 (2018).
Hayward, S. W., Cunha, G. R. & Dahiya, R. Normal development and carcinogenesis of the prostate. A unifying hypothesis. Ann. N. Y. Acad. Sci. 784, 50–62 (1996).
Acknowledgements
G.F. receives funding from the Fondation de France and the European Urology Scholarship Program. V.S. is supported by an MRC Clinical Research Training Fellowship (MR/S005897/1), the Mason Medical Research Foundation (project number: 558866) and acknowledges previous support from The Alan Turing Institute (EPSRC grant EP/N510129/1), the EACR (EACR Travel Fellowship) and UCL (Bogue Fellowship). E.S.C. is funded by a Barts Charity Lectureship (grant MGU045). S.H. is supported by a Movember-funded Prostate Cancer UK fellowship, TLD-PF16-004. A.N.A. is supported by the Medical Research Council (MR/P00184X/1) and an MRC Grand Challenge in Experimental Medicine Grant (MR/M003833/1). M.E. receives research support from the United Kingdom’s National Institute of Health Research (NIHR) UCLH/UCL Biomedical Centre. He was conferred NIHR Senior Investigator status in 2015.
Author information
Authors and Affiliations
Contributions
G.F., V.S., E.S.C. and S.H. researched data for the article. All authors contributed substantially to discussion of the content. G.F., V.S., E.S.C., S.H. and M.E. wrote the article. All authors reviewed and/or edited the manuscript before submission.
Corresponding author
Additional information
Competing interests
The authors declare no competing interests.
Peer review information
Nature Reviews Urology thanks A. Baniahmad, G. Ferbeyre and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fiard, G., Stavrinides, V., Chambers, E.S. et al. Cellular senescence as a possible link between prostate diseases of the ageing male. Nat Rev Urol 18, 597–610 (2021). https://doi.org/10.1038/s41585-021-00496-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41585-021-00496-8
This article is cited by
-
ERBB2-PTGS2 axis promotes intervertebral disc degeneration by regulating senescence of nucleus pulposus cells
BMC Musculoskeletal Disorders (2023)
-
Immune-related gene index predicts metastasis for prostate cancer patients undergoing radical radiotherapy
Experimental Hematology & Oncology (2023)
-
Glutathione Peroxidase 3 induced mitochondria-mediated apoptosis via AMPK /ERK1/2 pathway and resisted autophagy-related ferroptosis via AMPK/mTOR pathway in hyperplastic prostate
Journal of Translational Medicine (2023)
-
Prognostic and therapeutic potential of senescent stromal fibroblasts in prostate cancer
Nature Reviews Urology (2023)
-
Senescence-associated secretory phenotype constructed detrimental and beneficial subtypes and prognostic index for prostate cancer patients undergoing radical prostatectomy
Discover Oncology (2023)