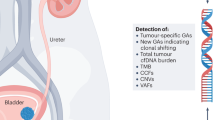
Abstract
The clinical presentation of renal cell cancer (RCC) is shifting towards incidental and early detection, creating new challenges in RCC diagnosis. Overtreatment might be reduced with the development of new diagnostic biomarkers to distinguish benign from malignant small renal masses (SRMs). Differently from tissue biopsies, liquid biopsies are obtained from a patient’s blood or urine and, therefore, are minimally invasive and suitable for longitudinal monitoring. The most promising types of liquid biopsy biomarkers for RCC diagnosis are circulating tumour cells, extracellular vesicles (EVs) and cell-free DNA. Circulating tumour cell assays have the highest specificity, with low processing time and costs. However, the biological characteristics and low sensitivity limit the use of these markers in SRM diagnostics. Cell-free DNA might complement the diagnosis of high-volume RCC, but the potential for clinical application in SRMs is limited. EVs have the highest biological abundance and the highest sensitivity in identifying low-volume disease; moreover, the molecular characteristics of these markers make EVs suitable for multiple analytical applications. Thus, currently, EV assays have the greatest potential for diagnostic application in RCC (including identification of SRMs). All these liquid biomarkers have potential in clinical practice, pending validation studies. Biomarker implementation will be needed to also improve characterization of RCC subtypes. Last, diagnostic biomarkers might be extended to prognostic or predictive applications.
Key points
-
The shift towards early renal cell cancer (RCC) diagnosis comes with a demand for novel biomarkers to face diagnostic challenges in the identification of small renal masses (SRMs) and characterization of RCC subtypes.
-
Liquid biopsy is less invasive than tumour biopsy, but, to date, no liquid biopsy biomarkers have been clinically approved for RCC diagnosis.
-
Circulating tumour cells (CTCs) seem less suitable than extracellular vesicles (EVs) for cancer detection in SRMs, owing to low epithelial cell adhesion molecule (EPCAM) expression in RCC and low biological abundance of CTCs in early stages of the disease.
-
Cell-free DNA seems to be more promising as a predictive biomarker of response to systemic metastatic RCC therapies than as a diagnostic biomarker owing to the low biological abundance of this marker in RCC, especially in SRMs.
-
EVs have the highest potential as diagnostic biomarkers in RCC, especially SRMs.
-
Further standardization and simplification are still required in all experimental RCC biomarker assays before liquid biopsy can be applied in clinical practice.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021).
Shuch, B. et al. Understanding pathologic variants of renal cell carcinoma: distilling therapeutic opportunities from biologic complexity. Eur. Urol. 67, 85–97 (2015).
Marconi, L. et al. Systematic review and meta-analysis of diagnostic accuracy of percutaneous renal tumour biopsy. Eur. Urol. 69, 660–673 (2016).
Capitanio, U. & Montorsi, F. Renal cancer. Lancet 387, 894–906 (2016).
Capitanio, U. et al. Epidemiology of renal cell carcinoma. Eur. Urol. 75, 74–84 (2019).
Rosiello, G., Larcher, A., Montorsi, F. & Capitanio, U. Renal cancer: overdiagnosis and overtreatment. World J. Urol. 39, 2821–2823 (2021).
Johnson, D. C. et al. Preoperatively misclassified, surgically removed benign renal masses: a systematic review of surgical series and United States population level burden estimate. J. Urol. 193, 30–35 (2015).
Fernando, A., Fowler, S. & O’Brien, T., British Association of Urological Surgeons. Nephron-sparing surgery across a nation — outcomes from the British Association of Urological Surgeons 2012 national partial nephrectomy audit. BJU Int. 117, 874–882 (2016).
Richard, P. O. et al. Renal tumor biopsy for small renal masses: a single-center 13-year experience. Eur. Urol. 68, 1007–1013 (2015).
Amaral, B. S. et al. Renal tumor biopsy: rationale to avoid surgery in small renal masses. Curr. Urol. Rep. 22, 46 (2021).
Ljungberg, B. et al. European association of urology guidelines on renal cell carcinoma: the 2022 update. Eur. Urol. 82, 399–410 (2022).
Ray, S., Cheaib, J. G. & Pierorazio, P. M. Active surveillance for small renal masses. Rev. Urol. 22, 9–16 (2020).
Farber, N. J. et al. Renal cell carcinoma: the search for a reliable biomarker. Transl. Cancer Res. 6, 620–632 (2017).
Rosellini, M. et al. Prognostic and predictive biomarkers for immunotherapy in advanced renal cell carcinoma. Nat. Rev. Urol. 20, 133–157 (2023).
Navani, V. & Heng, D. Y. C. Treatment selection in first-line metastatic renal cell carcinoma-the contemporary treatment paradigm in the age of combination therapy: a review. JAMA Oncol. 8, 292–299 (2022).
Rosellini, M. et al. Guiding treatment selection with immunotherapy compared to targeted therapy agents in patients with metastatic kidney cancer. Expert. Rev. Precis. Med. Drug. Dev. 7, 131–149 (2022).
Jonasch, E. et al. Belzutifan for renal cell carcinoma in von Hippel-Lindau disease. N. Engl. J. Med. 385, 2036–2046 (2021).
Powles, T. et al. Pembrolizumab versus placebo as post-nephrectomy adjuvant therapy for clear cell renal cell carcinoma (KEYNOTE-564): 30-month follow-up analysis of a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 23, 1133–1144 (2022).
Linehan, W. M. & Ricketts, C. J. The cancer genome atlas of renal cell carcinoma: findings and clinical implications. Nat. Rev. Urol. 16, 539–552 (2019).
Di Meo, A., Bartlett, J., Cheng, Y., Pasic, M. D. & Yousef, G. M. Liquid biopsy: a step forward towards precision medicine in urologic malignancies. Mol. Cancer 16, 80 (2017).
Raposo, G. et al. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 183, 1161–1172 (1996).
McKiernan, J. M. et al. The detection of renal carcinoma cells in the peripheral blood with an enhanced reverse transcriptase-polymerase chain reaction assay for MN/CA9. Cancer 86, 492–497 (1999).
Jahr, S. et al. DNA fragments in the blood plasma of cancer patients: quantitations and evidence for their origin from apoptotic and necrotic cells. Cancer Res. 61, 1659–1665 (2001).
Corro, C. et al. Detecting circulating tumor DNA in renal cancer: an open challenge. Exp. Mol. Pathol. 102, 255–261 (2017).
Fleischhacker, M. & Schmidt, B. Circulating nucleic acids (CNAs) and cancer — a survey. Biochim. Biophys. Acta 1775, 181–232 (2007).
Gorin, M. A. et al. Circulating tumour cells as biomarkers of prostate, bladder, and kidney cancer. Nat. Rev. Urol. 14, 90–97 (2017).
de Groot, A. E., Roy, S., Brown, J. S., Pienta, K. J. & Amend, S. R. Revisiting seed and soil: examining the primary tumor and cancer cell foraging in metastasis. Mol. Cancer Res. 15, 361–370 (2017).
van der Toom, E. E., Verdone, J. E., Gorin, M. A. & Pienta, K. J. Technical challenges in the isolation and analysis of circulating tumor cells. Oncotarget 7, 62754–62766 (2016).
Gong, J., Maia, M. C., Dizman, N., Govindarajan, A. & Pal, S. K. Metastasis in renal cell carcinoma: biology and implications for therapy. Asian J. Urol. 3, 286–292 (2016).
van der Toom, E. E. et al. Prostate-specific markers to identify rare prostate cancer cells in liquid biopsies. Nat. Rev. Urol. 16, 7–22 (2019).
Vona, G. et al. Isolation by size of epithelial tumor cells: a new method for the immunomorphological and molecular characterization of circulating tumor cells. Am. J. Pathol. 156, 57–63 (2000).
Reduzzi, C. et al. Development of a protocol for single-cell analysis of circulating tumor cells in patients with solid tumors. Adv. Exp. Med. Biol. 994, 83–103 (2017).
Blumke, K. et al. Detection of circulating tumor cells from renal carcinoma patients: experiences of a two-center study. Oncol. Rep. 14, 895–899 (2005).
Allard, W. J. et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin. Cancer Res. 10, 6897–6904 (2004).
Liu, S. et al. Combined cell surface carbonic anhydrase 9 and CD147 antigens enable high-efficiency capture of circulating tumor cells in clear cell renal cell carcinoma patients. Oncotarget 7, 59877–59891 (2016).
Sperger, J. M. et al. Integrated analysis of multiple biomarkers from circulating tumor cells enabled by exclusion-based analyte isolation. Clin. Cancer Res. 23, 746–756 (2017).
Cristofanilli, M. et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N. Engl. J. Med. 351, 781–791 (2004).
Cohen, S. J. et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J. Clin. Oncol. 26, 3213–3221 (2008).
Went, P. et al. Expression of epithelial cell adhesion molecule (EpCam) in renal epithelial tumors. Am. J. Surg. Pathol. 29, 83–88 (2005).
Zimpfer, A. et al. Prognostic and diagnostic implications of epithelial cell adhesion/activating molecule (EpCAM) expression in renal tumours: a retrospective clinicopathological study of 948 cases using tissue microarrays. BJU Int. 114, 296–302 (2014).
Zieglschmid, V. et al. Combination of immunomagnetic enrichment with multiplex RT-PCR analysis for the detection of disseminated tumor cells. Anticancer. Res. 25, 1803–1810 (2005).
Uemura, H. et al. MN/CA IX/G250 as a potential target for immunotherapy of renal cell carcinomas. Br. J. Cancer 81, 741–746 (1999).
Ye, Z. et al. Detecting and phenotyping of aneuploid circulating tumor cells in patients with various malignancies. Cancer Biol. Ther. 20, 546–551 (2019).
Wang, Z. L. et al. Dynamic changes of different phenotypic and genetic circulating tumor cells as a biomarker for evaluating the prognosis of RCC. Cancer Biol. Ther. 20, 505–512 (2019).
Nel, I. et al. Circulating tumor cell composition in renal cell carcinoma. PLoS One 11, e0153018 (2016).
Haga, N. et al. Perioperative detection of circulating tumor cells in radical or partial nephrectomy for renal cell carcinoma. Ann. Surg. Oncol. 27, 1272–1281 (2020).
Wu, C., Xu, C., Wang, G., Zhang, D. & Zhao, X. Noninvasive circulating tumor cell and urine cellular XPC (rs2228001, A2815C) and XRCC1 (rs25487, G1196A) polymorphism detection as an effective screening panel for genitourinary system cancers. Transl. Cancer Res. 8, 2803–2812 (2019).
Ge, L. et al. Clinical significance of circulating tumor cells detection in renal cell carcinoma with thrombus: a STROBE-compliant study. Medicine 99, e20615 (2020).
Gradilone, A. et al. Circulating tumor cells and “suspicious objects” evaluated through CellSearch® in metastatic renal cell carcinoma. Anticancer. Res. 31, 4219–4221 (2011).
Zhang, T. et al. Development of a novel c-MET-based CTC detection platform. Mol. Cancer Res. 14, 539–547 (2016).
Basso, U. et al. Prognostic role of circulating tumor cells in metastatic renal cell carcinoma: a large, multicenter, prospective trial. Oncologist 26, 740–750 (2021).
Bade, R. M. et al. Development and initial clinical testing of a multiplexed circulating tumor cell assay in patients with clear cell renal cell carcinoma. Mol. Oncol. 15, 2330–2344 (2021).
Bai, M. et al. Comparison of two detection systems for circulating tumor cells among patients with renal cell carcinoma. Int. Urol. Nephrol. 50, 1801–1809 (2018).
Xing, T., Wang, B., Song, Y., Zhang, S. & Ma, L. Candle soot-templated silica nanobiointerface chip for detecting circulating tumour cells from patients with urologic malignancies. RSC Adv. 8, 34566–34572 (2018).
Nayak, B. et al. Role of circulating tumor cells in patients with metastatic clear-cell renal cell carcinoma. Urol. Oncol. 39, 135.e9–135.e15 (2021).
El-Heliebi, A. et al. Are morphological criteria sufficient for the identification of circulating tumor cells in renal cancer? J. Transl. Med. 11, 214 (2013).
Wu, S. et al. Classification of circulating tumor cells by epithelial-mesenchymal transition markers. PLoS One 10, e0123976 (2015).
Naoe, M. et al. Development of a highly sensitive technique for capturing renal cell cancer circulating tumor cells. Diagnostics 9, 96 (2019).
Liu, M. et al. New applications of the acridine orange fluorescence staining method: screening for circulating tumor cells. Oncol. Lett. 13, 2221–2229 (2017).
Broncy, L. et al. Single-cell genetic analysis validates cytopathological identification of circulating cancer cells in patients with clear cell renal cell carcinoma. Oncotarget 9, 20058–20074 (2018).
Song, J. et al. Clinical significance of circulating tumour cells and Ki-67 in renal cell carcinoma. World J. Surg. Oncol. 19, 156 (2021).
Swaminathan, V. et al. Mechanical stiffness grades metastatic potential in patient tumor cells and in cancer cell lines. Cancer Res. 71, 5075–5080 (2011).
Kang, Y. T. et al. Cytopathological study of the circulating tumor cells filtered from the cancer patients’ blood using hydrogel-based cell block formation. Sci. Rep. 8, 15218 (2018).
Kim, T. H. et al. Detection of circulating tumour cells and their potential use as a biomarker for advanced renal cell carcinoma. Can. Urol. Assoc. J. 13, E285–E291 (2019).
Cappelletti, V. et al. Analysis of single circulating tumor cells in renal cell carcinoma reveals phenotypic heterogeneity and genomic alterations related to progression. Int. J. Mol. Sci. 21, 1475 (2020).
Lambros, M. B. et al. Single-cell analyses of prostate cancer liquid biopsies acquired by apheresis. Clin. Cancer Res. 24, 5635–5644 (2018).
Rupp, B., Ball, H., Wuchu, F., Nagrath, D. & Nagrath, S. Circulating tumor cells in precision medicine: challenges and opportunities. Trends Pharmacol. Sci. 43, 378–391 (2022).
Bluemke, K. et al. Detection of circulating tumor cells in peripheral blood of patients with renal cell carcinoma correlates with prognosis. Cancer Epidemiol. Biomark. Prev. 18, 2190–2194 (2009).
Cimadamore, A. et al. Emerging molecular technologies in renal cell carcinoma: liquid biopsy. Cancers 11, 196 (2019).
Jang, A. et al. Utility of circulating tumor DNA in monitoring treatment response to immune checkpoint inhibitors in patients with advanced genitourinary cancers. J. Clin. Oncol. 41, 721–721 (2023).
Basu, A. et al. Longitudinal detection of circulating tumor DNA in patients with advanced renal cell carcinoma. J. Clin. Oncol. 41, 715–715 (2023).
Colombo, M., Raposo, G. & Thery, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 30, 255–289 (2014).
Grange, C. et al. Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche. Cancer Res. 71, 5346–5356 (2011).
Yang, L., Wu, X., Wang, D., Luo, C. & Chen, L. Renal carcinoma cell-derived exosomes induce human immortalized line of Jurkat T lymphocyte apoptosis in vitro. Urol. Int. 91, 363–369 (2013).
Lindoso, R. S., Collino, F. & Camussi, G. Extracellular vesicles derived from renal cancer stem cells induce a pro-tumorigenic phenotype in mesenchymal stromal cells. Oncotarget 6, 7959–7969 (2015).
Xia, Y. et al. Negative regulation of tumor-infiltrating NK cell in clear cell renal cell carcinoma patients through the exosomal pathway. Oncotarget 8, 37783–37795 (2017).
Gai, C., Pomatto, M. A. C., Grange, C., Deregibus, M. C. & Camussi, G. Extracellular vesicles in onco-nephrology. Exp. Mol. Med. 51, 1–8 (2019).
De Toro, J., Herschlik, L., Waldner, C. & Mongini, C. Emerging roles of exosomes in normal and pathological conditions: new insights for diagnosis and therapeutic applications. Front. Immunol. 6, 203 (2015).
Dong, L. et al. Recent advances in extracellular vesicle research for urological cancers: from technology to application. Biochim. Biophys. Acta Rev. Cancer 1871, 342–360 (2019).
Coumans, F. A. W. et al. Methodological guidelines to study extracellular vesicles. Circ. Res. 120, 1632–1648 (2017).
Dong, L. et al. Comprehensive evaluation of methods for small extracellular vesicles separation from human plasma, urine and cell culture medium. J. Extracell. Vesicles 10, e12044 (2020).
Nakai, W. et al. A novel affinity-based method for the isolation of highly purified extracellular vesicles. Sci. Rep. 6, 33935 (2016).
Thery, C. et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for extracellular vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 7, 1535750 (2018).
Zieren, R. C. et al. Extracellular vesicle isolation from human renal cancer tissue. Med. Oncol. 37, 28 (2020).
Pugholm, L. H., Revenfeld, A. L., Sondergaard, E. K. & Jorgensen, M. M. Antibody-based assays for phenotyping of extracellular vesicles. Biomed. Res. Int. 2015, 524817 (2015).
Jang, S. C. et al. Mitochondrial protein enriched extracellular vesicles discovered in human melanoma tissues can be detected in patient plasma. J. Extracell. Vesicles 8, 1635420 (2019).
Jingushi, K. et al. Leukocyte-associated immunoglobulin-like receptor 1 promotes tumorigenesis in RCC. Oncol. Rep. 41, 1293–1303 (2019).
Himbert, D. et al. Characterization of CD147, CA9, and CD70 as tumor-specific markers on extracellular vesicles in clear cell renal cell carcinoma. Diagnostics 10, 1034 (2020).
Del Boccio, P. et al. A hyphenated microLC-Q-TOF-MS platform for exosomal lipidomics investigations: application to RCC urinary exosomes. Electrophoresis 33, 689–696 (2012).
Raimondo, F. et al. Differential protein profiling of renal cell carcinoma urinary exosomes. Mol. Biosyst. 9, 1220–1233 (2013).
Butz, H. et al. Exosomal microRNAs are diagnostic biomarkers and can mediate cell-cell communication in renal cell carcinoma. Eur. Urol. Focus 2, 210–218 (2016).
De Palma, G. et al. The three-gene signature in urinary extracellular vesicles from patients with clear cell renal cell carcinoma. J. Cancer 7, 1960–1967 (2016).
Song, S. et al. Urinary exosome miR-30c-5p as a biomarker of clear cell renal cell carcinoma that inhibits progression by targeting HSPA5. J. Cell Mol. Med. 23, 6755–6765 (2019).
Kuczler, M. D. et al. Advancements in the identification of EV derived mRNA biomarkers for liquid biopsy of clear cell renal cell carcinomas. Urology 160, 87–93 (2022).
Zhang, W. et al. MicroRNAs in serum exosomes as potential biomarkers in clear-cell renal cell carcinoma. Eur. Urol. Focus 4, 412–419 (2018).
Jingushi, K. et al. Extracellular vesicles isolated from human renal cell carcinoma tissues disrupt vascular endothelial cell morphology via azurocidin. Int. J. Cancer 142, 607–617 (2018).
Wang, X. et al. Serum exosomal miR-210 as a potential biomarker for clear cell renal cell carcinoma. J. Cell Biochem. 120, 1492–1502 (2018).
Dias, F. et al. Extracellular vesicles enriched in hsa-miR-301a-3p and hsa-miR-1293 dynamics in clear cell renal cell carcinoma patients: potential biomarkers of metastatic disease. Cancers 12, 1450 (2020).
Iliuk, A. et al. Plasma-derived extracellular vesicle phosphoproteomics through chemical affinity purification. J. Proteome Res. 19, 2563–2574 (2020).
Arance, E. et al. Determination of exosome mitochondrial DNA as a biomarker of renal cancer aggressiveness. Cancers 14, 199 (2021).
Xiao, C. T., Lai, W. J., Zhu, W. A. & Wang, H. MicroRNA derived from circulating exosomes as noninvasive biomarkers for diagnosing renal cell carcinoma. Onco Targets Ther. 13, 10765–10774 (2020).
He, X. et al. Circulating exosomal mRNA signatures for the early diagnosis of clear cell renal cell carcinoma. BMC Med. 20, 270 (2022).
Qian, H. et al. Diagnosis of urogenital cancer combining deep learning algorithms and surface-enhanced Raman spectroscopy based on small extracellular vesicles. Spectrochim. Acta A Mol. Biomol. Spectrosc. 281, 121603 (2022).
Linxweiler, J. & Junker, K. Extracellular vesicles in urological malignancies: an update. Nat. Rev. Urol. 17, 11–27 (2020).
Du, M. et al. Plasma exosomal miRNAs-based prognosis in metastatic kidney cancer. Oncotarget 8, 63703–63714 (2017).
Fujii, N. et al. Extracellular miR-224 as a prognostic marker for clear cell renal cell carcinoma. Oncotarget 8, 109877–109888 (2017).
Verzoni, E. et al. A platform for high-resolution immune liquid biopsy analysis to predict response in patients with renal cell carcinoma treated with nivolumab or cabozantinib: preliminary data from I-RENE trial (Meet-URO 8 study). J. Clin. Oncol. 41, 712–712 (2023).
Chen, G. et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 560, 382–386 (2018).
Santos, P. & Almeida, F. Exosome-based vaccines: history, current state, and clinical trials. Front. Immunol. 12, 711565 (2021).
Rezaie, J., Feghhi, M. & Etemadi, T. A review on exosomes application in clinical trials: perspective, questions, and challenges. Cell Commun. Signal. 20, 145 (2022).
Kim, M. S. et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine 12, 655–664 (2016).
Wu, H. et al. Bone marrow mesenchymal stem cells-derived exosomal microRNA-193a reduces cisplatin resistance of non-small cell lung cancer cells via targeting LRRC1. Cell Death Dis. 11, 801 (2020).
Celec, P., Vlkova, B., Laukova, L., Babickova, J. & Boor, P. Cell-free DNA: the role in pathophysiology and as a biomarker in kidney diseases. Expert. Rev. Mol. Med. 20, e1 (2018).
Mandel, P. & Metais, P. Nuclear acids in human blood plasma. C. R. Seances Soc. Biol. Fil. 142, 241–243 (1948).
Stroun, M., Anker, P., Lyautey, J., Lederrey, C. & Maurice, P. A. Isolation and characterization of DNA from the plasma of cancer patients. Eur. J. Cancer Clin. Oncol. 23, 707–712 (1987).
Schwarzenbach, H., Hoon, D. S. & Pantel, K. Cell-free nucleic acids as biomarkers in cancer patients. Nat. Rev. Cancer 11, 426–437 (2011).
Dasari, A. et al. ctDNA applications and integration in colorectal cancer: an NCI Colon and Rectal-Anal Task Forces whitepaper. Nat. Rev. Clin. Oncol. 17, 757–770 (2020).
Leest, P. V. et al. Comparison of circulating cell-free DNA extraction methods for downstream analysis in cancer patients. Cancers 12, 1222 (2020).
Ellinger, J. et al. Circulating mitochondrial DNA in serum: a universal diagnostic biomarker for patients with urological malignancies. Urol. Oncol. 30, 509–515 (2012).
Ellinger, J. et al. CpG island hypermethylation in cell-free serum DNA identifies patients with localized prostate cancer. Prostate 68, 42–49 (2008).
Vogelstein, B. et al. Cancer genome landscapes. Science 339, 1546–1558 (2013).
Torga, G. & Pienta, K. J. Patient-paired sample congruence between 2 commercial liquid biopsy tests. JAMA Oncol. 4, 868–870 (2018).
Teixeira, A. L., Dias, F., Gomes, M., Fernandes, M. & Medeiros, R. Circulating biomarkers in renal cell carcinoma: the link between microRNAs and extracellular vesicles, where are we now? J. Kidney Cancer VHL 1, 84–98 (2014).
Zeuschner, P., Linxweiler, J. & Junker, K. Non-coding RNAs as biomarkers in liquid biopsies with a special emphasis on extracellular vesicles in urological malignancies. Expert. Rev. Mol. Diagn. 20, 151–167 (2020).
Huang, G. et al. Combination of tumor suppressor miR-20b-5p, miR-30a-5p, and miR-196a-5p as a serum diagnostic panel for renal cell carcinoma. Pathol. Res. Pract. 216, 153152 (2020).
Wu, Y. et al. A serum-circulating long noncoding RNA signature can discriminate between patients with clear cell renal cell carcinoma and healthy controls. Oncogenesis 5, e192 (2016).
Zaporozhchenko, I. A., Ponomaryova, A. A., Rykova, E. Y. & Laktionov, P. P. The potential of circulating cell-free RNA as a cancer biomarker: challenges and opportunities. Expert. Rev. Mol. Diagn. 18, 133–145 (2018).
Tosar, J. P., Witwer, K. & Cayota, A. Revisiting extracellular RNA release, processing, and function. Trends Biochem. Sci. 46, 438–445 (2021).
Hauser, S. et al. Cell-free circulating DNA: diagnostic value in patients with renal cell cancer. Anticancer. Res. 30, 2785–2789 (2010).
Wan, J., Zhu, L., Jiang, Z. & Cheng, K. Monitoring of plasma cell-free DNA in predicting postoperative recurrence of clear cell renal cell carcinoma. Urol. Int. 91, 273–278 (2013).
Feng, G. et al. Quantification of plasma cell-free DNA in predicting therapeutic efficacy of sorafenib on metastatic clear cell renal cell carcinoma. Dis. Markers 34, 105–111 (2013).
Skrypkina, I. et al. Concentration and methylation of cell-free DNA from blood plasma as diagnostic markers of renal cancer. Dis. Markers 2016, 3693096 (2016).
de Martino, M., Klatte, T., Haitel, A. & Marberger, M. Serum cell-free DNA in renal cell carcinoma: a diagnostic and prognostic marker. Cancer 118, 82–90 (2012).
Lu, H. et al. Diagnostic and prognostic potential of circulating cell-free genomic and mitochondrial DNA fragments in clear cell renal cell carcinoma patients. Clin. Chim. Acta 452, 109–119 (2016).
Bettegowda, C. et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci. Transl. Med. 6, 224ra224 (2014).
Hauser, S., Zahalka, T., Fechner, G., Muller, S. C. & Ellinger, J. Serum DNA hypermethylation in patients with kidney cancer: results of a prospective study. Anticancer. Res. 33, 4651–4656 (2013).
Costa, V. L. et al. TCF21 and PCDH17 methylation: an innovative panel of biomarkers for a simultaneous detection of urological cancers. Epigenetics 6, 1120–1130 (2011).
Outeiro-Pinho, G. et al. MicroRNA-30a-5p(me): a novel diagnostic and prognostic biomarker for clear cell renal cell carcinoma in tissue and urine samples. J. Exp. Clin. Cancer Res. 39, 98 (2020).
Nuzzo, P. V. et al. Detection of renal cell carcinoma using plasma and urine cell-free DNA methylomes. Nat. Med. 26, 1041–1043 (2020).
Lasseter, K. et al. Plasma cell-free DNA variant analysis compared with methylated DNA analysis in renal cell carcinoma. Genet. Med. 22, 1366–1373 (2020).
Hahn, A. W. et al. Correlation of genomic alterations assessed by next-generation sequencing (NGS) of tumor tissue DNA and circulating tumor DNA (ctDNA) in metastatic renal cell carcinoma (mRCC): potential clinical implications. Oncotarget 8, 33614–33620 (2017).
Pal, S. K. et al. Evolution of circulating tumor DNA profile from first-line to subsequent therapy in metastatic renal cell carcinoma. Eur. Urol. 72, 557–564 (2017).
Maia, M. C. et al. Association of circulating tumor DNA (ctDNA) detection in metastatic renal cell carcinoma (mRCC) with tumor burden. Kidney Cancer 1, 65–70 (2017).
Yamamoto, Y. et al. Clinical significance of the mutational landscape and fragmentation of circulating tumor DNA in renal cell carcinoma. Cancer Sci. 110, 617–628 (2019).
Bacon, J. V. W. et al. Plasma circulating tumor DNA and clonal hematopoiesis in metastatic renal cell carcinoma. Clin. Genitourin. Cancer 18, 322–331 e322 (2020).
Smith, C. G. et al. Comprehensive characterization of cell-free tumor DNA in plasma and urine of patients with renal tumors. Genome Med. 12, 23 (2020).
Sumiyoshi, T. et al. Detection of von Hippel-Lindau gene mutation in circulating cell-free DNA for clear cell renal cell carcinoma. Cancer Sci. 112, 3363–3374 (2021).
Pascual, J. et al. ESMO recommendations on the use of circulating tumour DNA assays for patients with cancer: a report from the ESMO precision medicine working group. Ann. Oncol. 33, 750–768 (2022).
Acknowledgements
R.C.Z. discloses support for the research of this work from the Stichting Cure for Cancer Foundation, Amsterdam, the Netherlands. K.J.P. discloses support for the research of this work from the NCI [grant numbers U54CA143803, CA163124, CA093900 and CA143055] and the Prostate Cancer Foundation.
Author information
Authors and Affiliations
Contributions
R.C.Z. researched data for the article. All authors contributed substantially to discussion of the content. R.C.Z. and A.D.B. wrote the article. All authors reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Urology thanks Francesco Massari, Carmen Jeronimo and the other, anonymous, reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zieren, R.C., Zondervan, P.J., Pienta, K.J. et al. Diagnostic liquid biopsy biomarkers in renal cell cancer. Nat Rev Urol 21, 133–157 (2024). https://doi.org/10.1038/s41585-023-00818-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41585-023-00818-y