Abstract
The Genitourinary Pathology Society (GUPS) undertook a critical review of the recent advances in renal neoplasia, particularly focusing on the newly accumulated evidence post-2016 World Health Organization (WHO) classification. In the era of evolving histo-molecular classification of renal neoplasia, morphology is still key. However, entities (or groups of entities) are increasingly characterized by specific molecular features, often associated either with recognizable, specific morphologies or constellations of morphologies and corresponding immunohistochemical profiles. The correct diagnosis has clinical implications leading to better prognosis, potential clinical management with targeted therapies, may identify hereditary or syndromic associations, which may necessitate appropriate genetic testing. We hope that this undertaking will further facilitate the identification of these entities in practice. We also hope that this update will bring more clarity regarding the evolving classification of renal neoplasia and will further reduce the category of “unclassifiable renal carcinomas/tumors”. We propose three categories of novel entities: (1) “Novel entity”, validated by multiple independent studies; (2) “Emerging entity”, good compelling data available from at least two or more independent studies, but additional validation is needed; and (3) “Provisional entity”, limited data available from one or two studies, with more work required to validate them. For some entities initially described using different names, we propose new terminologies, to facilitate their recognition and to avoid further diagnostic dilemmas. Following these criteria, we propose as novel entities: eosinophilic solid and cystic renal cell carcinoma (ESC RCC), renal cell carcinoma with fibromyomatous stroma (RCC FMS) (formerly RCC with leiomyomatous or smooth muscle stroma), and anaplastic lymphoma kinase rearrangement-associated renal cell carcinoma (ALK-RCC). Emerging entities include: eosinophilic vacuolated tumor (EVT) and thyroid-like follicular renal cell carcinoma (TLFRCC). Finally, as provisional entities, we propose low-grade oncocytic tumor (LOT), atrophic kidney-like lesion (AKLL), and biphasic hyalinizing psammomatous renal cell carcinoma (BHP RCC).
Similar content being viewed by others
Introduction
In this work, the Genitourinary Pathology Society (GUPS) focused on the new developments in the classification of renal neoplasia, resulting in a proposal to recognize several novel, emerging, and provisional renal entities. This work is part of the GUPS kidney project aiming to provide updates on: (1) New developments in existing renal entities currently in the WHO classification (covered in a separate companion paper); and (2) New, emerging, and provisional entities. The second topic is covered in this paper. This GUPS project included 42 urologic pathologists from 11 countries; the work was organized in working groups (participants in the groups are listed in Table 1).
Traditional classifications of renal neoplasia have been based on criteria that include common morphology; a consistent (or specific) immunohistochemical profile; often a unifying molecular (genetic) profile; and established biologic behavior. In the current era of evolving histo-molecular classification, although morphology is still key, entities (or groups of entities) are increasingly characterized by specific molecular features. In some instances, these molecular features are associated either with recognizable, specific H&E morphologies, for example, in Succinate Dehydrogenase (SDH)-deficient renal cell carcinoma (RCC) or eosinophilic solid and cystic (ESC) RCC, or are seen in association with constellations of morphologies (or morphologic patterns) that are relatively congruent and suggestive of specific molecular abnormalities (for example, MiTF tumor family or fumarate hydratase (FH)-deficient RCC). The awareness and the recognition of these morphologies or patterns by the pathologist are critical, because the correct diagnosis may lead directly to better prognosis or more appropriate clinical management with an evolving array of targeted therapies, may help identify specific hereditary or syndromic associations, and initiate proband genetic testing. Elucidating these histo-molecular links may also help understand the disease processes of cancer and allow us to apply novel techniques to study them. Lastly, the process of entity “recognition” starts with their identification in practice, so that emerging entities can be flagged, studied, and validated, including their clinical implications for the patients.
In this section, we present several novel and evolving (‘emerging’ and ‘provisional’) renal entities. Despite the relative rarity, the correct diagnosis or at least suspicion for these tumors can be usually established on morphology, often by utilizing limited and appropriate immunohistochemistry (IHC), without resorting to exhaustive and often expensive testing. A summary of the key features of the new, emerging and provisional renal entities included in this paper, their current WHO status, and the GUPS proposals for their classification are shown in Table 2 (proposed categories are also included in the title of each entity).
In sum, we propose three categories of novel renal entities, based on the strength of the available evidence derived from a critical review of the literature and the expertise of the participants: (1) “Novel entity”, validated by multiple independent studies; (2) “Emerging entity”, good compelling data available from at least two or more independent studies, but still needs additional work and validation; and (3) “Provisional entity”, limited data available from one or two studies, with more work required to potentially validate them. For some entities that were initially described using different names, we propose new terminologies, to potentially facilitate their recognition and to avoid further diagnostic and terminological dilemmas that may be perpetuated in the future practice and studies.
The evolving spectrum of new entities has significantly reduced the category of “unclassified” renal carcinomas (or tumors). In fact, many of the newly recognized entities were previously part of the category of “unclassified (eosinophilic) carcinomas (or tumors)” [1, 2]. Some of these were identified in unclassified cancers with aggressive features [3], or in specific demographic groups (for example, in younger adults ≤35 years) [4], typically based on H&E morphology and using a limited IHC panel that included FH/ S-(2S-succino)-cysteine (2SC), SDHB and CK20 [4]. The recent recognition of SDH-deficient RCC and FH-deficient RCC has also prompted institutional re-evaluation of cases originally diagnosed as “oncocytoma”, “papillary RCC”, or “unclassified RCC”, by applying routine IHC screening to retrospectively identify such cases and to establish institutional incidence of some novel tumors [5]. It should be acknowledged, however, that there still exist a group of currently “unclassifiable renal carcinomas/tumors” that do not fit into any of the previously recognized or proposed “novel/emerging” renal subtypes. The diagnosis of these cases is typically one of exclusion, after appropriate differential consideration and work-up. Although one may have an impression that all “unclassified tumors” are by default high-grade [3], that is certainly not the case, as such tumors may also exist in the “low-grade” spectrum, which are expected to behave indolently, based on their small size, low-stage, and “low-grade” features. Thus, when reporting a tumor as “unclassified”, it behooves pathologists to provide additional descriptive characterization or to use a diagnostic note, to convey to the clinicians if the tumor potentially belongs to the “high-grade” or “low-grade” RCC spectrum, and to document the entities considered and ruled out in the differential diagnosis, based on the diagnostic work-up.
It is also worth noting that tuberous sclerosis complex (TSC)/MTOR mutations are more commonly found in some novel or emerging entities, which in a context of certain morphology and IHC profile, support the diagnosis. However, patients with TSC show a more heterogeneous spectrum of renal neoplasia, considered TSC-related [6, 7], but there are also sporadic counterparts with very similar (or identical) morphology, found in patients without TSC [8,9,10,11,12,13,14,15]. Such mutations are also typically found in angiomyolipoma (AML) or PEComa. The two most recognizable subtypes are “ESC RCC” and “RCC with fibromyomatous (or leiomyomatous) stroma”, which are identical to their more common sporadic counterparts. As more studies highlight the somatic tumors with underlying TSC1, TSC2, or MTOR mutations, it is becoming clear that the morphologic spectrum of these seemingly low-risk oncocytic tumors is broad and their classification is still evolving. These mutations, however, are neither specific nor pathognomonic, and have been described in various other renal tumors. For example, MTOR activation/mutation has been well documented in some common renal tumors, such as metastatic clear cell RCC or papillary RCC [16, 17], chromophobe RCC [18, 19], as well as in acquired cystic disease-associated RCC [20], and in some “unclassified aggressive RCCs” [3]. Although the concept of “TSC/MTOR renal tumor family” may be appealing, it is evident that tumors bearing such mutations represent a diverse group and exhibit different biologic behavior, and lumping them together is currently unjustifiable. More work is needed to address questions on the exact role of the TSC/MTOR mutations, if they are driver or passenger mutations, as well as whether they are somatic or germline mutations. Therefore, we currently do not recommend designating “TSC-associated RCC” as a specific subtype, but rather recommend the classification of each individual RCC with these mutations based on the most appropriate category. The key features of the renal entities included in this paper that demonstrate specific TSC/MTOR mutations are summarized in Table 2.
Eosinophilic solid and cystic renal cell carcinoma (Novel entity)
ESC RCC has been recently proposed as a novel entity based on a constellation of clinical, pathologic, immunohistochemical, and molecular features [8,9,10,11, 13, 14, 21]. The great majority of ESC RCC are sporadic, but rare tumors with identical morphology have also been reported in patients with TSC [6, 7, 22, 23]. ESC RCC are typically solitary and low-stage tumors, but occasional multifocal and bilateral cases have been documented [4, 8, 9, 11, 24]. They are mostly found in females, with a broad age range distribution, including pediatric patients [4, 8, 9, 11]. The incidence is currently unknown as many cases have been previously diagnosed as “unclassified RCCs” or other entities. The great majority of ESC RCC are indolent tumors, but rare cases have been reported with metastases, justifying the “carcinoma” designation, and the necessity for an ongoing patient surveillance [8,9,10,11, 24, 25].
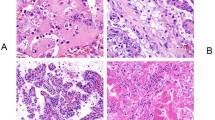
The morphologic features and the relatively consistent immunohistochemical profile are generally sufficient for the diagnosis of ESC RCC (Fig. 1). ESC RCC is a well-circumscribed and non-encapsulated tumor, grossly demonstrating solid and cystic growth. Macrocysts are a key gross feature, although rare cases demonstrate only microscopic cysts [8, 9]. The solid tumor areas exhibit diffuse, compact acinar, or nested growth. The cells show eosinophilic, voluminous cytoplasm, with readily recognizable, coarse cytoplasmic granules (‘stippling’) [8, 9]. The nuclei are round to oval, but the nucleoli are generally not prominent. The cyst lining typically has a hobnail arrangement. Small clusters of admixed foamy histiocytes and lymphocytes are often found. Focal papillary growth can also be present, and morphologic variations may be seen in individual cases, including “clear cell” areas, insular or tubular growth, and clusters of multinucleated cells [8, 9]. Psammoma bodies are found in about half of the cases [8, 9].
This is a grossly solid, gray-tan tumor with macrocysts (A). Microscopically (B), it typically shows solid or compact acinar growth, with cells exhibiting voluminous eosinophilic cytoplasm; the cells in the solid parts and in the cyst walls (center) are identical. Scattered foamy histocytes (C) are often found. Coarse cytoplasmic granules (stippling) (D) are easily seen on higher magnification. Using immunohistochemistry, this tumor typically shows CK20 reactivity (E), with negative CK7 (F).
IHC reactivity for CK20 (either diffuse or focal) is present in about 85% of cases, usually accompanied by a negative or very focally positive CK7 (in less than a quarter of cases) [8, 9]. Negative CK20 can however be seen in 10–15% of cases, but a greater degree of CK7 reactivity is unusual [8, 9]. Other positive stains include PAX8, AE1/AE3, CK8/18, and vimentin. Cathepsin K was found to be reactive in the majority of ESC RCC [4, 11]. Negative stains typically include CD117, CAIX, and HMB45; melan A may be positive in occasional cases [8, 9, 11].
Molecular evaluation of sporadic ESC RCC by next-generation sequencing (NGS) has demonstrated consistent and mutually exclusive, somatic bi-allelic mutations in the TSC genes, TSC1 and TSC2 [9, 10, 21, 26]. The TSC1/2 genes inhibit the mTOR complex and when mutated lead to activation of mTORC1 and dysregulation of downstream cellular pathways including cell growth and proliferation [11, 21, 27]. Notably, a complete response of metastatic disease has been documented with everolimus, a rapamycin analog, in a 13-year-old female non-TSC patient with multifocal ESC RCC, and a confirmed somatic TSC2 mutation [11]. The frequent TSC gene mutations in ESC RCC, taken together with the clinical, morphologic, and IHC features, indicate that ESC RCC represents a distinct histologic and molecular entity. A subset of renal neoplasms previously classified as “oncocytoid RCC after neuroblastoma” likely represent ESC RCC based on histologic and molecular evidence [11, 28].
Renal cell carcinoma with fibromyomatous stroma (Novel entity)
RCC with fibromyomatous stroma (RCC FMS) was included as an “emerging/provisional” entity in the 2016 WHO classification as a “RCC with (angio)leiomyomatous stroma” [29]. In the past, there was a debate whether RCC FMS represents a separate entity or a group of RCCs with overlapping morphologies [13, 30,31,32,33,34]. Accordingly, various names have been used to refer to the RCCs that exhibited clear cells and prominent smooth muscle and fibromatous stroma [13]. Recent studies have demonstrated that RCC FMS indeed represents a distinct entity that can be separated from other RCCs that exhibit clear cells, as well as tubulopapillary morphology and smooth muscle/fibromatous stroma, such as clear cell RCC and CCPRCC [15, 35]. RCC FMS has subtle but distinct morphological features that can aid in distinguishing it from clear cell RCC and CCPRCC in the great majority of the cases, as illustrated in Table 3 [15]. The distinction between RCC FMS, clear cell RCC and CCPRCC may indeed be challenging, especially on renal biopsy, when the tissue is limited and may not sample the salient features, such as the stroma or other morphologic features that may distinguish between these entities. Such a distinction may be important in deciding, for example, if partial/radical nephrectomy should be pursued vs. surveillance in patients who are older or have significant comorbidities. In this scenario, pursuing an immunohistochemical evaluation using CK7, CAIX, CD10, and high molecular weight cytokeratin, as outlined in Table 3, may help distinguish between these entities.
RCC FMS is typically a solid tumor, composed of an epithelial component, admixed with a stromal fibromuscular component that may be particularly abundant at the periphery. The stromal component has been found to be polyclonal [36], and its extent can vary [15]. Microscopically, the epithelial component forms tumor nodules composed of elongated and frequently branching tubules, lined by clear or mildly eosinophilic cells containing voluminous cytoplasm. Focal papillary morphology is also frequently present (Fig. 2). Diffuse CK7 positivity is typical and is generally required for the diagnosis; reactivity for CAIX and CD10 are also usually present [15].
This tumor is characterized by nests or papillary formations composed of cells with clear cytoplasm (A), dispersed in a fibromuscular stroma (B). At high magnification, these tumors have abundant apical cytoplasm, usually clear to pale eosinophilic (C). CK7 is typically positive in over half of the tumor cells (D).
Molecular analysis of these tumors demonstrated recurrent mutations involving the TSC/MTOR pathway. A subset of tumors with similar morphology has shown mutations involving ELOC (previously referred to as TCEB1), typically associated with monosomy 8 [15, 30, 33, 37]. Both molecular subgroups uniformly lack VHL or chromosome 3p alterations [15, 31, 32, 35]. It is currently debated whether TSC/MTOR and ELOC mutated RCC FMS should be grouped together, based on their shared and overlapping morphology and common CK7 reactivity, despite the differing molecular alterations. Interestingly, one case with confirmed monosomy 8 has recently been shown to exhibit both an ELOC deletion as well as a TSC1 mutation [15]. Finally, in addition to the more common RCC FMS that are sporadic, essentially identical tumors have been found in patients with TSC, suggesting the existence of hereditary and sporadic counterparts of this tumor [15]. Based on the relatively limited follow-up, most RCC FMS with TSC/MTOR mutations have demonstrated an indolent biological behavior [15]; however, lymph node metastases have been reported in rare cases associated with TSC [6]. Although the initial report on ELOC (TCEB1)-associated RCC FMS suggested indolent behavior [37], a recent report demonstrated that aggressive behavior can also occur, with two cases showing distant metastases [38].
Current evidence supports the recognition of RCC FMS as a novel subtype of RCC with morphologic, IHC, and molecular characteristics distinct from clear cell RCC and CCPRCC. It is however recommended that for cases where the morphology and IHC do not provide a definitive diagnosis, to perform additional molecular evaluation for possible VHL gene abnormalities, as well as ELOC (TCEB1) and TSC/MTOR, so that possible differences between these tumors can be more clearly elucidated.
Eosinophilic vacuolated tumor (Emerging entity)
This is an emerging renal tumor with unique morphology, relatively consistent immunoprofile and distinct molecular genetic features. We propose the name “eosinophilic vacuolated tumor” (EVT) for the entity initially described in two recent studies as “high-grade oncocytic tumor” by He et al. [39] and “sporadic RCC with eosinophilic and vacuolated cytoplasm” by Chen et al. [40]. This tumor emerged from the spectrum of “unclassified eosinophilic tumors”, and does not fit into any of the currently recognized renal tumor categories. In addition to the sporadic cases described in the initial studies, one case was also documented in association with TSC [12]. It is possible that some tumors previously described as “chromophobe-like”, or demonstrating “microvesicular” or “vaculolated” cytoplasm in TSC patients may represent this type of neoplasm [6, 7]. Tumors with similar morphology harboring TSC mutations have also been included in two recent studies of “eosinophilic tumors with TSC mutations” [11, 41]. The features of this entity have also been recently summarized [14]. So far, no aggressive cases have been documented, although the reported cases had a relatively short follow-up.
These tumors are more common in women (M:F = 1:2.5) and are found in patients of a broad age range from 25 to 73 years (mean 50.9, median 54 years) [39, 40]. The tumors ranged from 1.5 to 7 cm (mean 3.4 cm), and all were pT1 stage. On follow-up, all tumors demonstrated indolent course (mean follow-up 28 months) [39].
These tumors are well-circumscribed but unencapsulated, with solid, tan, or mahogany brown cut surface and typically do not show cysts. They demonstrate solid to nested growth with focal tubulocystic areas. The tumor cells exhibit eosinophilic (oncocytic) cytoplasm, with frequent, often large intracytoplasmic vacuoles (Fig. 3A–C). The cells also show prominent membranes and round to oval nuclei, often with enlarged nucleoli that would typically correspond to WHO/ISUP grade 3. Normal renal tubules and thick-walled vessels are frequently found at the periphery.
These tumors demonstrate solid growth with frequent and prominent intracytoplasmic vacuoles (A). The cells in some areas are however mostly oncocytic (B). Cells have voluminous cytoplasm, enlarged round to oval nuclei with often very prominent nucleoli (C). Cathepsin K (D) is positive on immunohistochemistry (focal or diffuse) in almost all cases.
The neoplastic cells are immunoreactive for PAX8, AE1/AE3, and antimitochondrial antigen, and often for cathepsin K (Fig. 3D), CD117, and CD10. CK7 is variable, from negative to focally positive (typically only in scattered cells), and SDHB and FH are normal/positive (“retained”). Negative stains include vimentin, CK20, HMB45, Melan-A, and TFE3. On electron microscopy numerous intracytoplasmic mitochondria were found [14].
The copy number alterations in EVT included losses of chromosomes 1 and 19 [39, 40]. None of the evaluated cases demonstrated TFE3 or TFEB rearrangements [39]. Sequencing analysis of five cases identified somatic TSC2 inactivating mutations or MTOR activating mutation [40]. Both cases with MTOR activating mutations also had a loss of chromosome 1.
Despite some morphologic similarities to renal oncocytoma, ChRCC, and t(6;11) translocation RCC, these tumors do not fit into these recognized categories. The high degree of cytologic and nuclear atypia and voluminous cytoplasmic vacuoles distinguish these cases from oncocytoma and ChRCC. EVT indeed shares molecular similarities with ESC RCC that include the TSC2 and TSC1 mutations and activation of the mTOR pathway; similarly, both tumors can be seen in TSC patients, although they are more common sporadically. EVT can, however, be distinguished from ESC RCC based on morphology, because it does not show a cystic component, typically shows cytoplasmic large vacuoles (usually not seen in ESC RCC), and lacks the coarse cytoplasmic granularity seen in ESC RCC. On IHC, EVT often demonstrates CD117+/vimentin-/CK20- profile, different from the CD117−/vimentin+/CK20+ profile seen in ESC RCC.
Low-grade oncocytic tumor (Provisional entity)
Low-grade oncocytic tumor (LOT) of the kidney has recently emerged as a provisional entity from the spectrum of eosinophilic renal tumors with “oncocytic” features that pose diagnostic challenges [42, 43]. LOT has likely been previously diagnosed as either oncocytoma, unclassified oncocytic tumor, or as eosinophilic ChRCC. This is compounded by the lack of stringent diagnostic criteria in the WHO 2016 classification for eosinophilic ChRCC, which only require it to be “almost purely eosinophilic” [44]. Based on two published studies (36 cases) [42, 43], all LOTs were single and relatively small, tan to brown tumors, occurring in a non-syndromic setting, and showed a fairly consistent morphology and immunoprofile. To date, these neoplasms have not been found to have aggressive behavior (hence “tumor” is preferred to “carcinoma”). LOT can be recognized by morphology based on its predominant solid architecture, bland eosinophilic (“oncocytic”) cells, with “low-grade”, round to oval nuclei, lacking more prominent irregularities and often showing delicate perinuclear halos. Sharply delineated edematous stromal areas, containing loosely connected strands of tumor cells or individual cells are frequently found centrally (Fig. 4) [42, 43]. Some cases mimic SDH-deficient RCC but lack cytoplasmic vacuoles and show retained expression of SDHB.
A common feature in this tumor is edematous stroma with loosely connected cells that appear stretched out (A) rather than arranged in the typical round nests of oncocytoma. At high magnification, the cytology is usually bland and monotonous, with or without some perinuclear clearing (B). Diffuse CK7 labeling is typical (C), which differs from the rare positive cells usually seen in oncocytoma. KIT (CD117) staining (D) is typically negative, although mast cells may be positive, as in this example.
This neoplasm typically shows diffuse IHC reactivity for CK7 and negative KIT (CD117), which also aids in its distinction from oncocytoma and ChRCC [42, 43]. This profile would be unusual for oncocytoma (usually only scattered cells CK7 positive, with diffuse KIT positive) or classic ChRCC (often diffuse CK7 and diffuse KIT positive) [45]. Using electron microscopy, LOT demonstrates abundant and closely packed cytoplasmic mitochondria, similar to oncocytoma [14].
The molecular studies of these tumors are limited and array CGH evaluation has generally found diploid pattern, without complete chromosomal losses or gains, and only deletions of 19p13, 19q13, and 1p36 [42]. A morphologically similar group of low-grade oncocytic tumors exhibiting diffuse CK7 on IHC, demonstrated mutations in TSC1 or TSC2, suggesting that this is another type of renal neoplasm characterized by alterations in the TSC/MTOR pathway [41]. Additional, morphologically similar tumors were identified in the TCGA ChRCC cohort, labeled as “eosinophilic ChRCC” that lacked any copy number alterations and demonstrated TSC/MTOR mutations [46]. Most recently, 5 cases labeled “eosinophilic chromophobe-like renal tumors”, morphologically identical to LOT, demonstrated strikingly low or null FOXI1 mRNA expression (in contrast to renal oncocytoma and ChRCC), distinct transcriptomic profiles and clustering attributes, frequent mutations involving the mTOR signaling pathway, and absence of losses of chromosomes 1, 2, 6, 10, and 17, further supporting the conclusion that LOT represents a distinct entity [47]. In another recent study utilizing NGS methodology, MTOR mutations have also been found in two tumors with identical morphology (originally considered “eosinophilic ChRCC”), that showed a negative CD117 phenotype, very low FOXI1 and diploid chromosomal pattern [48].
ALK rearrangement-associated renal cell carcinoma (Novel entity)
Anaplastic lymphoma kinase rearrangement-associated RCC (ALK-RCC) was previously listed in the 2016 WHO classification as an “emerging/provisional” entity [29]. The newly accumulated data however supports the conclusion that ALK-RCC represents a novel and genetically distinct entity, typically demonstrating a heterogeneous morphology (Fig. 5). ALK-RCC is characterized by an ALK gene rearrangement resulting in fusion with various partner genes leading to aberrant ALK activation and formation of oncogenic chimeric proteins. Despite the morphologic diversity, ALK rearrangement can be consistently identified either by IHC, fluorescence in situ hybridization (FISH) or by sequencing methods. ALK-RCC has also attracted clinical interest because of the availability of targeted ALK inhibitor therapies [49], with documented response to therapy in some aggressive ALK-RCC cases [50, 51]. Since the first reports in 2011 [52, 53] about 40 cases have been documented [13, 14, 50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69]. Although the majority of ALK-RCCs appear indolent, they may exhibit malignant behavior, including metastatic disease and death, documented in about 25% of reported cases.
Microscopically, some areas show papillary and trabecular growth (A); note the mucinous background. Solid areas may also be present (B), whereas other areas contain tubulocystic growth (C), and focal signet-ring cells (D). Focal and distinct metanephric adenoma-like morphology can be seen in this case, which was in continuity with the other patterns (E). ALK immunohistochemical staining is positive in the neoplastic cells (F), including both in the heterogeneous and metanephric adenoma-like area of the same tumor.
ALK-RCCs are solitary, non-infiltrative, solid, or solid–cystic tumors, with white-gray to yellow and variegated cut surface. ALK-RCC demonstrates equal gender distribution and so far have not been reported in a syndromic or hereditary setting, or in association with other non-renal tumors harboring ALK rearrangement. ALK-RCC has been found in patients of wide age range. One group includes pediatric African-American patients with sickle cell trait, with tumors typically occurring in the renal medulla and exhibiting VCL-ALK and TPM3-ALK fusions [52, 53, 57]. Pediatric ALK-RCCs exhibit morphologic similarities to adult renal medullary carcinoma and collecting duct carcinoma, with relatively uniform, solid or reticular/syncytial/tubular growth, delicate vascular network, often admixed with marked lymphoplasmacytic inflammatory infiltrate and stromal desmoplasia [52, 53, 57, 59, 61]. The neoplastic cells in the pediatric ALK-RCCs may also show prominent vacuolization. The adult group of ALK-RCC are usually cortical and show a heterogeneous morphology with multiple admixed growth patterns present in a single case, including papillary (or pseudopapillary), solid, tubular or tubulocystic, cribriform, trabecular, spindle cell, and signet-ring individual cells [69]. The cells have eosinophilic cytoplasm and variable cytomorphologies, including rhabdoid, vacuolated, pleomorphic giant cell, and small cell (metanephric adenoma-like) morphology. The morphologic spectrum has been recently expanded to include ALK-RCC with metanephric adenoma-like and mucinous tubular and spindle RCC-like morphologies [69, 70]. Psammomatous calcifications may also be present, as well as coagulative necrosis [55, 62, 69]. Another important and frequent finding is an extensive mucinous/myxoid background, including intracytoplasmic mucin [54, 62, 65, 69]. Thus, ALK-RCC should be considered in the differential of any RCC with heterogeneous, difficult to classify morphology with mucinous/myxoid background, before labeling these cases “unclassified RCC” [69].
Patients with ALK-RCC demonstrate diffuse cytoplasmic and membranous ALK protein expression using IHC. The use of next generation antibody clones (for example, 5A4 or D5F3) is highly sensitive and specific; however, given the rarity of this tumor, demonstration of a confirmatory ALK rearrangement by FISH or other molecular means is highly desirable for definitive diagnosis. The immunohistochemical profile of ALK-RCC is otherwise non-specific and may include reactivity for PAX8, CK7, 34βE12, AMACR, and vimentin. SMARCB1 (INI1), SDHB, and FH are normal (positive/retained) [13, 14, 57, 69]. Melanocytic markers, such as melan A, HMB45, S100, and cathepsin K, as well as CK20 and GATA3 are all negative [62, 69]. Immunoreactivity for TFE3, but without TFE3-rearrangement by FISH, has been reported in some pediatric cases [57, 59, 61, 64] and in two adult ALK-RCCs with TPM3-ALK fusion [65, 69].
Molecular studies have identified a number of ALK fusion partners. VCL-ALK has been found in pediatric African-American patients with sickle trait [52, 53, 57, 59]. A recent report demonstrated a VCL-ALK fusion in a 57-year-old Chinese woman, but without “clinical evidence” of sickle cell trait, and hemoglobin electrophoresis was not reported [68]. Other ALK fusion gene partners include: TPM3, EML4, STRN, HOOK1, and PLEKHA7 [54, 59,60,61,62, 65, 66, 70]. Three new ALK partners have most recently been identified in a multi-institutional study of 12 ALK-RCCs: CLIP1, KIF5B, and KIAA1217 [69].
Thyroid-like follicular renal cell carcinoma (Emerging entity)
Thyroid-like follicular RCC (TLFRCC) was included as an emerging/provisional RCC subtype in the 2016 WHO classification, characterized as resembling “thyroid parenchyma, with follicles and colloid” (Fig. 6) [29]. Since the first report in 2004, 34 cases have been published in the English literature [71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93], with additional examples documented in the non-English, mainly Chinese, literature.
TLFRCC is more commonly found in females (M:F = 1:1.8) and has a wide age distribution (10–83 years, mean 40.4 years). It is usually detected incidentally and typically presents at a low stage. Lymph node and distant metastases were documented in about 10% of cases [78, 80, 82, 87, 91]. The majority of TLFRCCs behaved indolently [78, 80, 91].
Grossly, TLFRCCs are solid, well-circumscribed tumors. The mean size is 4.7 cm (range, 1–16.5 cm). On microscopy, they demonstrate a distinct follicular pattern with variably sized follicles, arranged back-to-back and filled with colloid-like material. The follicles are lined by a single layer of cuboidal or low columnar epithelium, with WHO/ISUP grade 2 or 3 nuclei. The majority of tumors showed a pure follicular architecture, with some variations, including branching, resulting in a focal papillary pattern [85, 91, 93]; sarcomatoid differentiation has also been reported [82].
On IHC, all TLFRCC are negative for TTF1 and thyreogobulin, which distinguish them from metastatic thyroid follicular carcinomas, the main differential diagnosis. The majority of cases were positive for CK7 (89%) and variable reactivity has been reported for RCC (14%), AMACR (17%), CD10 (23%), and CK20 (40%), but so far a consistent immunophenotype has not emerged.
Very limited genetic data are available on TLFRCC. No consistent chromosomal copy number changes nor recurrent genetic alterations have been found [72, 82, 91, 94, 95].
Based on the collected evidence so far, it remains unclear if TLFRCC represents a separate and distinct RCC entity, because the limited number of studied tumors lacked a distinct IHC profile and typical molecular characteristics. Although the follicular architecture is considered a defining feature, it is not unique to TLFRCC and may be seen in other RCC types, as a focal or even diffuse finding, including PRCC, clear cell RCC, FH-deficient RCC, tubulocystic RCC, and also in oncocytoma and mixed epithelial and stromal tumor [96,97,98,99]. Some examples demonstrating both papillary and follicular patterns probably represent variants of PRCC [96, 97], although they have been sometimes classified as TLFRCC [85, 93]. Atrophic kidney-like tumor/lesion has also been misdiagnosed as TLFRCC [100], and two tumors reported as TLFRCC did not have the typical morphology of TLFRCC, based on the provided images [101, 102]. Thus, more studies are needed to further characterize this tumor, especially its molecular features, utilizing cases diagnosed by strict morphological criteria and exclusive follicular morphology, after excluding possible mimickers [72].
Atrophic kidney-like lesion (Provisional entity)
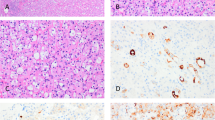
“Atrophic kidney-like lesion” (AKLL) has been described in two case series and one larger study [99, 103, 104]. Two additional cases also likely represent this lesion, despite being designated “thyroid-like follicular carcinoma”, another emerging renal entity [100, 105]. AKLL presents as a circumscribed and compact brown nodule in the renal cortex, typically showing a thick capsule. It displays a well-developed follicular architecture with varying sized follicles, containing dense eosinophilic secretions and scattered microcalcifications, due to psammoma bodies or coarse amorphous deposits (Fig. 7). The epithelial lining of the follicles is composed of flat and atrophic cells, focally showing a hobnail appearance; detached cells floating in the eosinophilic luminal fluid are common. The follicle lining cells show an immunophenotype of the normal glomerular podocytes (WT1+/PAX8−/CK7−). Intraluminal glomerular tufts can be highlighted by PAS and by endothelial markers, suggesting that the “follicles” actually represent cystically dilated glomeruli. The tissue between the follicles is composed of atrophic tubules with dense collagenous stroma, or small collapsed glomeruli. Although the etiology of this lesion is debated, particularly because the presentation of a solitary encapsulated mass may suggest a neoplasm, the presence of glomerular structures argues strongly against the neoplastic origin and supports the conclusion that this may be a localized/segmental form of non-neoplastic glomerulocystic and atrophic tubular change [104]. All documented cases of AKLL to date have exhibited benign behavior [99, 103, 104].
Biphasic hyalinizing psammomatous renal cell carcinoma (Provisional entity)
“Biphasic hyalinizing psammomatous renal cell carcinoma” (BHP RCC) with features mimicking MiTF family translocation RCC (particularly TFEB rearranged tumors) was recently reported in a series of eight cases, the majority in men [106]. These tumors were unencapsulated and predominantly showed solid and variable papillary architecture. They were composed of a biphasic neoplastic population, with smaller cells clustering around basement membrane material and forming pseudorosettes, as typically seen in TFEB RCC t(6:11); the second cell population consisted of larger cells with pale cytoplasm. The smaller cells also formed spindle cell foci not associated with basement membrane material. Another common feature was focal tubulopapillary growth associated with basement membrane material, resulting in a glomeruloid appearance. The stroma was typically sclerotic and psammoma bodies were frequently seen (Fig. 8). The neoplastic cells were usually reactive for PAX8, CK7, HNF1-beta, and EMA; all cases were negative for GATA3, cathepsin K, melan A, inhibin, SF-1, and WT1. All tested cases were also negative for TFE3 and TFEB rearrangements by break-apart FISH. An underlying molecular feature was that all cases had a somatic mutations of the neurofibromin 2 (NF2) gene [106]. However, such NF2 mutations are not specific for this entity and have been found in other established RCC types, as well as in 18% of “unclassified and aggressive RCCs”, often in combinations with other genes [3]. A subset of such tumors may represent BHP RCC but how many remains to be defined.
Conclusions
In this collective work by the GUPS, we have summarized the current knowledge and the newly accumulated evidence since the WHO 2016 classification of renal tumors, on several novel, emerging and provisional renal entities to hopefully bring more clarity regarding the evolving histo-molecular classification of renal neoplasia. To increase their recognition and to promote better understanding among clinicians of their known biologic potential, we recommend that pathologists include a comment or a note at the end of the report, when signing out such cases. We hope that the recognition of the increasing spectrum of novel, emerging and provisional renal entities, will allow practicing pathologists and clinicians to translate these developments into more accurate diagnosis, management, and improved prognostication for individual patients.
Change history
16 March 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41379-021-00775-0
References
Perrino CM, Grignon DJ, Williamson SR, Idrees MT, Eble JN, Cheng L. Morphological spectrum of renal cell carcinoma, unclassified: an analysis of 136 cases. Histopathology. 2018;72:305–19.
Andeen NK, Qu X, Antic T, Tykodi SS, Fang M, Tretiakova MS. Clinical utility of chromosome genomic array testing for unclassified and advanced-stage renal cell carcinomas. Arch Pathol Lab Med. 2019;143:494–504.
Chen YB, Xu J, Skanderup AJ, Dong Y, Brannon AR, Wang L, et al. Molecular analysis of aggressive renal cell carcinoma with unclassified histology reveals distinct subsets. Nat Commun. 2016;7:13131.
Li Y, Reuter VE, Matoso A, Netto GJ, Epstein JI, Argani P. Re-evaluation of 33 ‘unclassified’ eosinophilic renal cell carcinomas in young patients. Histopathology. 2018;72:588–600.
Gupta S, Swanson AA, Chen YB, Lopez T, Milosevic D, Kipp BR, et al. Incidence of succinate dehydrogenase and fumarate hydratase-deficient renal cell carcinoma based on immunohistochemical screening with SDHA/SDHB and FH/2SC. Hum Pathol. 2019;91:114–22.
Guo J, Tretiakova MS, Troxell ML, Osunkoya AO, Fadare O, Sangoi AR, et al. Tuberous sclerosis-associated renal cell carcinoma: a clinicopathologic study of 57 separate carcinomas in 18 patients. Am J Surg Pathol. 2014;38:1457–67.
Yang P, Cornejo KM, Sadow PM, Cheng L, Wang M, Xiao Y, et al. Renal cell carcinoma in tuberous sclerosis complex. Am J Surg Pathol. 2014;38:895–909.
Trpkov K, Hes O, Bonert M, Lopez JI, Bonsib SM, Nesi G, et al. Eosinophilic, solid, and cystic renal cell carcinoma: clinicopathologic study of 16 unique, sporadic neoplasms occurring in women. Am J Surg Pathol. 2016;40:60–71.
Trpkov K, Abou-Ouf H, Hes O, Lopez JI, Nesi G, Comperat E, et al. Eosinophilic solid and cystic renal cell carcinoma (ESC RCC): further morphologic and molecular characterization of ESC RCC as a distinct entity. Am J Surg Pathol. 2017;41:1299–308.
Parilla M, Kadri S, Patil SA, Ritterhouse L, Segal J, Henriksen KJ, et al. Are sporadic eosinophilic solid and cystic renal cell carcinomas characterized by somatic tuberous sclerosis gene mutations? Am J Surg Pathol. 2018;42:911–7.
Palsgrove DN, Li Y, Pratilas CA, Lin MT, Pallavajjalla A, Gocke C, et al. Eosinophilic solid and cystic (ESC) renal cell carcinomas harbor TSC mutations: molecular analysis supports an expanding clinicopathologic spectrum. Am J Surg Pathol. 2018;42:1166–81.
Trpkov K, Bonert M, Gao Y, Kapoor A, He H, Yilmaz A, et al. High-grade oncocytic tumour (HOT) of kidney in a patient with tuberous sclerosis complex. Histopathology. 2019;75:440–2.
Trpkov K, Hes O. New and emerging renal entities: a perspective post-WHO 2016 classification. Histopathology. 2019;74:31–59.
Siadat F, Trpkov K. ESC, ALK, HOT and LOT: three letter acronyms of emerging renal entities knocking on the door of the WHO classification. Cancers. 2020;12:168.
Shah RB, Stohr BA, Tu ZJ, Gao Y, Przybycin CG, Nguyen J, et al. “Renal Cell Carcinoma With Leiomyomatous Stroma” Harbor Somatic Mutations of TSC1, TSC2, MTOR, and/or ELOC (TCEB1): Clinicopathologic and Molecular Characterization of 18 Sporadic Tumors Supports a Distinct Entity. Am J Surg Pathol. 2020;44:571–81.
Schultz L, Chaux A, Albadine R, Hicks J, Kim JJ, De Marzo AM, et al. Immunoexpression status and prognostic value of mTOR and hypoxia-induced pathway members in primary and metastatic clear cell renal cell carcinomas. Am J Surg Pathol. 2011;35:1549–56.
Kwiatkowski DJ, Choueiri TK, Fay AP, Rini BI, Thorner AR, de Velasco G, et al. Mutations in TSC1, TSC2, and MTOR are associated with response to rapalogs in patients with metastatic renal cell carcinoma. Clin Cancer Res. 2016;22:2445–52.
Roldan-Romero JM, Santos M, Lanillos J, Caleiras E, Anguera G, Maroto P, et al. Molecular characterization of chromophobe renal cell carcinoma reveals mTOR pathway alterations in patients with poor outcome. Mod Pathol. 2020;33:2580–90.
Chaux A, Albadine R, Schultz L, Hicks J, Carducci MA, Argani P, et al. Dysregulation of the mammalian target of rapamycin pathway in chromophobe renal cell carcinomas. Hum Pathol. 2013;44:2323–30.
Shah A, Lal P, Toorens E, Palmer MB, Schwartz L, Vergara N, et al. Acquired cystic kidney disease-associated renal cell carcinoma (ACKD-RCC) harbor recurrent mutations in KMT2C and TSC2 genes. Am J Surg Pathol. 2020;44:1479–86.
Mehra R, Vats P, Cao X, Su F, Lee ND, Lonigro R, et al. Somatic bi-allelic loss of TSC genes in eosinophilic solid and cystic renal cell carcinoma. Eur Urol. 2018;74:483–6.
Schreiner A, Daneshmand S, Bayne A, Countryman G, Corless CL, Troxell ML. Distinctive morphology of renal cell carcinomas in tuberous sclerosis. Int J Surg Pathol. 2010;18:409–18.
Park JH, Lee C, Chang MS, Kim K, Choi S, Lee H, et al. Molecular characterization and putative pathogenic pathways of tuberous sclerosis complex-associated renal cell carcinoma. Transl Oncol. 2018;11:962–70.
Tretiakova MS. Eosinophilic solid and cystic renal cell carcinoma mimicking epithelioid angiomyolipoma: series of 4 primary tumors and 2 metastases. Hum Pathol. 2018;80:65–75.
McKenney JK, Przybycin C, Trpkov K, Magi-Galluzzi C. Eosinophilic solid and cystic (ESC) renal cell carcinomas have metastatic potential. Histopathology. 2018;72:1066–7.
Eich ML, Tregnago AC, Faraj SF, Palsgrove DN, Fujita K, Bezerra SM, et al. Insulin-like growth factor-1 receptor expression in upper tract urothelial carcinoma. Virchows Arch. 2019;474:21–7.
Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–93.
Falzarano SM, McKenney JK, Montironi R, Eble JN, Osunkoya AO, Guo J, et al. Renal cell carcinoma occurring in patients with prior neuroblastoma: a heterogenous group of neoplasms. Am J Surg Pathol. 2016;40:989–97.
Moch H, Humphrey PA, Ulbright TM, Reuter VE. WHO classification of tumours of the urinary system and male genital organs. 4th ed. Lyon, France: International Agency for Research on Cancer; 2016.
Parilla M, Alikhan M, Al-Kawaaz M, Patil S, Kadri S, Ritterhouse LL, et al. Genetic underpinnings of renal cell carcinoma with leiomyomatous stroma. Am J Surg Pathol. 2019;43:1135–44.
Peckova K, Grossmann P, Bulimbasic S, Sperga M, Perez Montiel D, Daum O, et al. Renal cell carcinoma with leiomyomatous stroma–further immunohistochemical and molecular genetic characteristics of unusual entity. Ann Diagn Pathol. 2014;18:291–6.
Petersson F, Martinek P, Vanecek T, Pivovarcikova K, Peckova K, Ondic O, et al. Renal'ma: a group of tumors with indistinguishable histopathologic features, but 2 distinct genetic profiles: next-generation sequencing analysis of 6 cases negative for aberrations related to the VHL gene. Appl Immunohistochem Mol Morphol 2018;26:192–7.
Lan TT, Keller-Ramey J, Fitzpatrick C, Kadri S, Taxy JB, Segal JP, et al. Unclassified renal cell carcinoma with tubulopapillary architecture, clear cell phenotype, and chromosome 8 monosomy: a new kid on the block. Virchows Arch. 2016;469:81–91.
Williamson SR. Renal cell carcinomas with a mesenchymal stromal component: what do we know so far? Pathology. 2019;51:453–62.
Williamson SR, Cheng L, Eble JN, True LD, Gupta NS, Wang M, et al. Renal cell carcinoma with angioleiomyoma-like stroma: clinicopathological, immunohistochemical, and molecular features supporting classification as a distinct entity. Mod Pathol. 2015;28:279–94.
Petersson F, Branzovsky J, Martinek P, Korabecna M, Kruslin B, Hora M, et al. The leiomyomatous stroma in renal cell carcinomas is polyclonal and not part of the neoplastic process. Virchows Arch. 2014;465:89–96.
Hakimi AA, Tickoo SK, Jacobsen A, Sarungbam J, Sfakianos JP, Sato Y, et al. TCEB1-mutated renal cell carcinoma: a distinct genomic and morphological subtype. Mod Pathol. 2015;28:845–53.
DiNatale RG, Gorelick AN, Makarov V, Blum KA, Silagy AW, Freeman B, et al. Putative drivers of aggressiveness in TCEB1-mutant renal cell carcinoma: an emerging entity with variable clinical course. Eur Urol Focus. 2019 [Epub ahead of print].
He H, Trpkov K, Martinek P, Isikci OT, Maggi-Galuzzi C, Alaghehbandan R, et al. “High-grade oncocytic renal tumor”: morphologic, immunohistochemical, and molecular genetic study of 14 cases. Virchows Arch. 2018;473:725–38.
Chen YB, Mirsadraei L, Jayakumaran G, Al-Ahmadie HA, Fine SW, Gopalan A, et al. Somatic mutations of TSC2 or MTOR characterize a morphologically distinct subset of sporadic renal cell carcinoma with eosinophilic and vacuolated cytoplasm. Am J Surg Pathol. 2019;43:121–31.
Tjota M, Chen H, Parilla M, Wanjari P, Segal J, Antic T. Eosinophilic renal cell tumors With a TSC and MTOR gene mutations are morphologically and immunohistochemically heterogenous: clinicopathologic and molecular study. Am J Surg Pathol. 2020;44:943–54.
Trpkov K, Williamson SR, Gao Y, Martinek P, Cheng L, Sangoi AR, et al. Low-grade oncocytic tumor of kidney (CD117 negative, cytokeratin 7 positive): a distinct entity? Histopathology. 2019;75:174–84.
Guo Q, Liu N, Wang F, Guo Y, Yang B, Cao Z, et al. Characterization of a distinct low-grade oncocytic renal tumor (CD117-negative and cytokeratin 7-positive) based on a tertiary oncology center experience: the new evidence from China. Virchows Arch. 2020. [Epub ahead of print].
Paner G, Amin M, Moch H, Storke lS. Chromophobe renal cell carcinoma. In: Moch HHP, Ulbright TM, Reuter VE, editors. WHO classification of tumours of the urinary system and male genital organs: Lyon: International Agency for Research on Cancer; 2016. p. 27–8.
Wobker SE, Williamson SR. Modern pathologic diagnosis of renal oncocytoma. J Kidney Cancer Vhl. 2017;4:1–12.
Davis CF, Ricketts CJ, Wang M, Yang L, Cherniack AD, Shen H, et al. The somatic genomic landscape of chromophobe renal cell carcinoma. Cancer Cell. 2014;26:319–30.
Tong K, Hu Z. FOXI1 expression in chromophobe renal cell carcinoma and renal oncocytoma: a study of The Cancer Genome Atlas transcriptome-based outlier mining and immunohistochemistry. Virchows Arch. 2020. [Epub ahead of print].
Skala SL, Wang X, Zhang Y, Mannan R, Wang L, Narayanan SP, et al. Next-generation RNA sequencing-based biomarker characterization of chromophobe renal cell carcinoma and related oncocytic neoplasms. Eur Urol. 2020;78:63–74.
Hallberg B, Palmer RH. Mechanistic insight into ALK receptor tyrosine kinase in human cancer biology. Nat Rev Cancer. 2013;13:685–700.
Pal SK, Bergerot P, Dizman N, Bergerot C, Adashek J, Madison R, et al. Responses to alectinib in ALK-rearranged papillary renal cell carcinoma. Eur Urol 2018;74:124–8.
Tao J, Wei G, Patel R, Fagan P, Hao X, Bridge J, et al. ALK fusions in renal cell carcinoma: response to entrectinib. JCO Precis Oncol. 2018;2:1–8.
Marino-Enriquez A, Ou WB, Weldon CB, Fletcher JA, Perez-Atayde AR. ALK rearrangement in sickle cell trait-associated renal medullary carcinoma. Genes Chromosomes Cancer. 2011;50:146–53.
Debelenko LV, Raimondi SC, Daw N, Shivakumar BR, Huang D, Nelson M, et al. Renal cell carcinoma with novel VCL-ALK fusion: new representative of ALK-associated tumor spectrum. Mod Pathol. 2011;24:430–42.
Sugawara E, Togashi Y, Kuroda N, Sakata S, Hatano S, Asaka R, et al. Identification of anaplastic lymphoma kinase fusions in renal cancer: large-scale immunohistochemical screening by the intercalated antibody-enhanced polymer method. Cancer. 2012;118:4427–36.
Sukov WR, Hodge JC, Lohse CM, Akre MK, Leibovich BC, Thompson RH, et al. ALK alterations in adult renal cell carcinoma: frequency, clinicopathologic features and outcome in a large series of consecutively treated patients. Mod Pathol. 2012;25:1516–25.
Lee C, Park JW, Suh JH, Nam KH, Moon KC. ALK-positive renal cell carcinoma in a large series of consecutively resected korean renal cell carcinoma patients. Korean J Pathol. 2013;47:452–7.
Smith NE, Deyrup AT, Marino-Enriquez A, Fletcher JA, Bridge JA, Illei PB, et al. VCL-ALK renal cell carcinoma in children with sickle-cell trait: the eighth sickle-cell nephropathy? Am J Surg Pathol. 2014;38:858–63.
Ryan C, Mayer N, Cunningham J, Hislop G, Pratt N, Fleming S. Increased ALK1 copy number and renal cell carcinoma-a case report. Virchows Arch. 2014;464:241–5.
Cajaiba MM, Jennings LJ, Rohan SM, Perez-Atayde AR, Marino-Enriquez A, Fletcher JA, et al. ALK-rearranged renal cell carcinomas in children. Genes Chromosomes Cancer. 2016;55:442–51.
Cajaiba MM, Jennings LJ, George D, Perlman EJ. Expanding the spectrum of ALK-rearranged renal cell carcinomas in children: identification of a novel HOOK1-ALK fusion transcript. Genes Chromosomes Cancer. 2016;55:814–7.
Thorner PS, Shago M, Marrano P, Shaikh F, Somers GR. TFE3-positive renal cell carcinomas are not always Xp11 translocation carcinomas: report of a case with a TPM3-ALK translocation. Pathol Res Pract. 2016;212:937–42.
Kusano H, Togashi Y, Akiba J, Moriya F, Baba K, Matsuzaki N, et al. Two cases of renal cell carcinoma harboring a novel STRN-ALK fusion gene. Am J Surg Pathol. 2016;40:761–9.
Jeanneau M, Gregoire V, Desplechain C, Escande F, Tica DP, Aubert S, et al. ALK rearrangements-associated renal cell carcinoma (RCC) with unique pathological features in an adult. Pathol Res Pract. 2016;212:1064–6.
Oyama Y, Nishida H, Kusaba T, Kadowaki H, Arakane M, Daa T, et al. A case of anaplastic lymphoma kinase-positive renal cell carcinoma coincident with Hodgkin lymphoma. Pathol Int. 2017;67:626–31.
Yu W, Wang Y, Jiang Y, Zhang W, Li Y. Genetic analysis and clinicopathological features of ALK-rearranged renal cell carcinoma in a large series of resected Chinese renal cell carcinoma patients and literature review. Histopathology. 2017;71:53–62.
Bodokh Y, Ambrosetti D, Kubiniek V, Tibi B, Durand M, Amiel J, et al. ALK-TPM3 rearrangement in adult renal cell carcinoma: Report of a new case showing loss of chromosome 3 and literature review. Cancer Genet. 2018;221:31–7.
Yang J, Dong L, Du H, Li XB, Liang YX, Liu GR. ALK-TPM3 rearrangement in adult renal cell carcinoma: a case report and literature review. Diagn Pathol. 2019;14:112.
Wang XT, Fang R, Ye SB, Zhang RS, Li R, Wang X, et al. Targeted next-generation sequencing revealed distinct clinicopathologic and molecular features of VCL-ALK RCC: A unique case from an older patient without clinical evidence of sickle cell trait. Pathol Res Pract. 2019;215:152651.
Kuroda N, Trpkov K, Gao Y, Tretiakova M, Liu YJ, Ulamec M, et al. ALK rearranged renal cell carcinoma (ALK-RCC): a multi-institutional study of twelve cases with identification of novel partner genes CLIP1, KIF5B and KIAA1217. Mod Pathol. 2020;33:2564–79.
Hang JF, Chung HJ, Pan CC. ALK-rearranged renal cell carcinoma with a novel PLEKHA7-ALK translocation and metanephric adenoma-like morphology. Virchows Arch. 2020;476:921–9.
Agrawal V, Neyaz Z, Kapoor R. Thyroid-like follicular carcinoma of the kidney with oncocytic cells: a case report and review of metastatic and non-metastatic tumors. Int J Surg Pathol. 2020;28:913–7.
Amin MB, Gupta R, Ondrej H, McKenney JK, Michal M, Young AN, et al. Primary thyroid-like follicular carcinoma of the kidney: report of 6 cases of a histologically distinctive adult renal epithelial neoplasm. Am J Surg Pathol. 2009;33:393–400.
Chen F, Wang Y, Wu X, Zhu Y, Jiang X, Chen S, et al. Clinical characteristics and pathology of thyroid-like follicular carcinoma of the kidney: report of 3 cases and a literature review. Mol Clin Oncol. 2016;4:143–50.
Alessandrini L, Fassan M, Gardiman MP, Guttilla A, Zattoni F, Galletti TP. Thyroid-like follicular carcinoma of the kidney: report of two cases with detailed immunohistochemical profile and literature review. Virchows Arch. 2012;461:345–50.
Cavalcante A, Kuwano AY, Costa-Matos A, Spanholi EF, Souza T, Mascarenhas FM. Thyroid-like follicular carcinoma of the kidney - Case report. Urol Case Rep. 2017;15:36–8.
Chougule A, Bal A, Das A, Nayak B. Thyroid-like follicular renal cell carcinoma: an emerging morphological variant. Pathology. 2014;46:657–60.
Dawane R, Grindstaff A, Parwani AV, Brock T, White WM, Nodit L. Thyroid-like follicular carcinoma of the kidney: one case report and review of the literature. Am J Clin Pathol. 2015;144:796–804.
Dhillon J, Tannir NM, Matin SF, Tamboli P, Czerniak BA, Guo CC. Thyroid-like follicular carcinoma of the kidney with metastases to the lungs and retroperitoneal lymph nodes. Hum Pathol. 2011;42:146–50.
Dhillon J, Mohanty SK, Krishnamurthy S. Cytologic diagnosis of thyroid-like follicular carcinoma of the kidney: a case report. Diagn Cytopathol 2014;42:273–7.
Dong L, Huang J, Huang L, Shi O, Liu Q, Chen H, et al. Thyroid-like follicular carcinoma of the kidney in a patient with skull and meningeal Metastasis: a unique case report and review of the literature. Medicine. 2016;95:e3314.
Ghaouti M, Roquet L, Baron M, Pfister C, Sabourin JC. Thyroid-like follicular carcinoma of the kidney: a case report and review of the literature. Diagn Pathol. 2014;9:186.
Jenkins TM, Rosenbaum J, Zhang PJ, Schwartz LE, Nayak A, Cooper K, et al. Thyroid-like follicular carcinoma of the kidney with extensive sarcomatoid differentiation: a case report and review of the literature. Int J Surg Pathol. 2019;27:678–83.
de Jesus LE, Fulgencio C, Leve T, Dekermacher S. Thyroid-like follicular carcinoma of the kidney presenting on a 10 year-old prepubertal girl. Int Braz J Urol. 2019;45:834–42.
Khoja HA, Almutawa A, Binmahfooz A, Aslam M, Ghazi AA, Almaiman S. Papillary thyroid carcinoma-like tumor of the kidney: a case report. Int J Surg Pathol. 2012;20:411–5.
Li C, Dong H, Fu W, Qi M, Han B. Thyroid-like follicular carcinoma of the kidney and papillary renal cell carcinoma with thyroid-like feature: comparison of two cases and literature review. Ann Clin Lab Sci. 2015;45:707–12.
Lin YZ, Wei Y, Xu N, Li XD, Xue XY, Zheng QS, et al. Thyroid-like follicular carcinoma of the kidney: a report of two cases and literature review. Oncol Lett. 2014;7:1796–802.
Vicens RA, Balachandran A, Guo CC, Vikram R. Multimodality imaging of thyroid-like follicular renal cell carcinoma with lung metastases, a new emerging tumor entity. Abdom Imaging. 2014;39:388–93.
Malde S, Sheikh I, Woodman I, Fish D, Bilagi P, Sheriff MK. Primary thyroid-like follicular renal cell carcinoma: an emerging entity. Case Rep Pathol. 2013;2013:687427.
Volavsek M, Strojan-Flezar M, Mikuz G. Thyroid-like follicular carcinoma of the kidney in a patient with nephrolithiasis and polycystic kidney disease: a case report. Diagn Pathol. 2013;8:108.
Wang H, Yu J, Xu Z, Li G. Clinicopathological study on thyroid follicular carcinoma-like renal tumor related to serious hypertension: Case report and review of the literature. Medicine. 2017;96:e6419.
Sterlacci W, Verdorfer I, Gabriel M, Mikuz G. Thyroid follicular carcinoma-like renal tumor: a case report with morphologic, immunophenotypic, cytogenetic, and scintigraphic studies. Virchows Arch. 2008;452:91–5.
Wu WW, Chu JT, Nael A, Rezk SA, Romansky SG, Shane L. Thyroid-like follicular carcinoma of the kidney in a young patient with history of pediatric acute lymphoblastic leukemia. Case Rep Pathol. 2014;2014:313974.
Zhang Y, Yang J, Zhang M, Meng Z, Song W, Yang L, et al. Thyroid follicular carcinoma-like renal tumor: a case report and literature review. Medicine. 2018;97:e10815.
Jung SJ, Chung JI, Park SH, Ayala AG, Ro JY. Thyroid follicular carcinoma-like tumor of kidney: a case report with morphologic, immunohistochemical, and genetic analysis. Am J Surg Pathol. 2006;30:411–5.
Fanelli GN, Fassan M, Dal Moro F, Soligo M, Munari G, Zattoni F, et al. Thyroid-like follicular carcinoma of the kidney: the mutational profiling reveals a BRAF wild type status. Pathol Res Pract. 2019;215:152532.
Ohe C, Kuroda N, Pan CC, Yang XJ, Hes O, Michal M, et al. A unique renal cell carcinoma with features of papillary renal cell carcinoma and thyroid-like carcinoma: a morphological, immunohistochemical and genetic study. Histopathology. 2010;57:494–7.
Fadare O, Lam S, Rubin C, Renshaw IL, Nerby CL. Papillary renal cell carcinoma with diffuse clear cells and thyroid-like macrofollicular areas. Ann Diagn Pathol. 2010;14:284–91.
Tretiakova MS, Kehr EL, Gore JL, Tykodi SS. Thyroid-like follicular renal cell carcinoma arising within benign mixed epithelial and stromal tumor. Int J Surg Pathol. 2020;28:80–6.
Hes O, de Souza TG, Pivovarcikova K, Grossmann P, Martinek P, Kuroda N, et al. Distinctive renal cell tumor simulating atrophic kidney with 2 types of microcalcifications. Report of 3 cases. Ann Diagn Pathol. 2014;18:82–8.
Muscara MJ, Simper NB, Gandia E. Thyroid-like follicular carcinoma of the kidney. Int J Surg Pathol. 2017;25:73–7.
Ko JJ, Grewal JK, Ng T, Lavoie JM, Thibodeau ML, Shen Y, et al. Whole-genome and transcriptome profiling of a metastatic thyroid-like follicular renal cell carcinoma. Cold Spring Harb Mol Case Stud. 2018;4:a0031137.
Rao V, Menon S, Bakshi G, Prakash G, Agarwal A, Desai S. Thyroid-like follicular carcinoma of the kidney with low-grade sarcomatoid component: a hitherto undescribed case. Int J Surg Pathol. 2020. https://doi.org/10.1177/1066896920940406.
Oshiro Y, Hida AI, Tamiya S, Toyoshima S, Kuroda N, Hes O, et al. Bilateral atrophic kidney-like tumors. Pathol Int 2014;64:478–80.
Herlitz L, Hes O, Michal M, Tretiakova M, Reyes-Mugica M, Nguyen JK, et al. “Atrophic Kidney”-like Lesion: clinicopathologic series of 8 cases supporting a benign entity distinct from thyroid-like follicular carcinoma. Am J Surg Pathol. 2018;42:1585–95.
Berens S, Vogt P, Alkadhi H, Berger N, Moch H. [Thyroid-like follicular carcinoma of the kidney: a separate tumor entity?]. Pathologe. 2014;35:83–7.
Argani P, Reuter VE, Eble JN, Vlatkovic L, Yaskiv O, Swanson D, et al. Biphasic hyalinizing psammomatous renal cell carcinoma (BHP RCC): a distinctive neoplasm associated with somatic NF2 mutations. Am J Surg Pathol. 2020;44:901–16.
Acknowledgements
We wish to thank Ruby Reyes for her excellent technical assistance in the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: There was a typo in the heading of figure 5.
Rights and permissions
About this article
Cite this article
Trpkov, K., Williamson, S.R., Gill, A.J. et al. Novel, emerging and provisional renal entities: The Genitourinary Pathology Society (GUPS) update on renal neoplasia. Mod Pathol 34, 1167–1184 (2021). https://doi.org/10.1038/s41379-021-00737-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-021-00737-6
This article is cited by
-
Convolutional neural networks for the differentiation between benign and malignant renal tumors with a multicenter international computed tomography dataset
Insights into Imaging (2024)
-
A pilot radiometabolomics integration study for the characterization of renal oncocytic neoplasia
Scientific Reports (2023)
-
Evaluation of an institutional series of low-grade oncocytic tumor (LOT) of the kidney and review of the mutational landscape of LOT
Virchows Archiv (2023)
-
Mutated ASXL1 upregulates mTOR expression in renal cell carcinoma with fibromyomatous stroma
Virchows Archiv (2023)
-
Renal phenotypes correlate with genotypes in unrelated individuals with tuberous sclerosis complex in China
Orphanet Journal of Rare Diseases (2022)